Influence of Sediment Characteristics on Heavy Metal Toxicity in an Urban Marsh
by Carolyn S. Bentivegna, Joy-Elaine Alfano, Sean M. Bugel, and Katarzyna Czechowicz
Department of Biology, Seton Hall University, South Orange, NJ 07079
Abstract
Remediation and protection of urban wetlands are gaining public support as the contribution of these wetlands to biodiversity, and their importance to local fisheries and wildlife, become better understood. When developing remediation strategies, it is important to consider the key parameters that influence availability and toxicity of contaminants to which plant and animal life are exposed. Kearny Marsh is an important component of the Hackensack Meadowlands, which, located as they are in one of the most populated metropolitan areas in the United States, have been subjected to urban encroachment. We undertook studies to determine what sediment and detritus characteristics might be influencing heavy metal toxicity in Kearny Marsh. Toxicity parameters included sediment grain size, percentage of total organic carbon (%TOC), SEM–AVS, and heavy metal concentrations in whole sediment and detritus. These parameters were correlated with ten-day survival and growth, under laboratory conditions, of the aquatic larvae of Chironomus riparius (midge fly) in order to determine what factors were having the most effect on toxicity. Data showed that both sediment and detritus were highly contaminated with heavy metals. High metal levels in detritus had a significantly negative effect on the survival and growth of Chironomus larvae. Conversely, high iron-to-metal ratios in both sediment and detritus were correlated with reduced toxicity. The %TOC in sediments was linked to larval growth in October but not in June. SEM–AVS and grain size were not good indicators of toxicity. We conclude that detritus and iron could prove to be important factors for controlling and remediating heavy metal toxicity in Kearny Marsh and other wetlands in highly urbanized areas.
Key words: Acid volatile sulfides; Chironomus; detritus; Hackensack Meadowlands; heavy metals; iron; remediation; sediment; total organic carbon; toxicity testing; wetland.
Introduction
Kearny Marsh, in New Jersey, is a 320-acre freshwater wetland within the 8,400-acre estuary system known as the Hackensack Meadowlands. It has been heavily impacted by urban sprawl, including landfill construction within the marsh, housing and commercial development around the marsh, and the 1970 extension of the New Jersey Turnpike (Interstate Highway 95) through the marsh. Extension of the turnpike involved the installation of a dike that separated the marsh from the tidal flow of the Hackensack River and left it with no natural inlet or outlet of water. This allowed contaminants to settle into and concentrate in sediments. Because of its size and uniqueness as a freshwater wetland, Kearny Marsh is an important component of the Hackensack Meadowlands, and there is now increased interest in preserving the remaining acreage and improving its environmental health. Wetlands are highly productive ecosystems that usually provide an abundance of food for a diversity of fish and bird species, and eventually, through the food chain, for humans. Although Kearny Marsh has been damaged due to urbanization, marshes have incredible regenerative ability in general, and intervention at Kearny might improve its productivity.
Kearny Marsh sediments are severely contaminated with heavy metals (Langan Engineering and Environmental Services, Inc., 1999), so improving the marsh might require costly sediment remediation. However, the extent to which Kearny Marsh sediments are actually toxic has not been investigated. A critical factor for sediment toxicity is contaminant bioavailability—the degree to which contaminants can be taken up by plants and animals (Ankley, Di Toro, Hansen & Berry, 1996). Sediment parameters that affect heavy metal bioavailability include cation exchange capacity (CEC), total organic carbon (TOC), iron (Fe) and manganese (Mn) oxides, as well as the relationship between acid volatile sulfides (AVS) and simultaneously extracted metals (SEM). Following is a brief explanation of these parameters.
Cation exchange capacity is based on the surface area of sediment grain particles available for binding cations, such as hydrogen (H+) and free metal ions (e.g., Mn+2). Sediments with a high percentage of small grains, such as silt and clay, have high surface-to-volume ratios and can adsorb more heavy metals than sediments composed of large grains, such as sand. Total organic carbon is added to sediments primarily through the decomposition of plant and animal matter. Organic carbon can directly adsorb heavy metals from solutions applied to sediments (Liber et al., 1996). However, it can also contain heavy metals accumulated by plants that have been exposed to contaminated sediment during their lifetimes (Peltier, Webb & Gaillard, 2003). Nonetheless, high percentages of organic matter and/or small grains in sediment are generally associated with reduced heavy metal bioavailability and toxicity (Ankley et al., 1996).
Iron and Mn are major heavy metal components of both soil and sediment and can exist as dissolved ions or various precipitates, such as oxyhydroxides (oxides) and sulfides. Both Fe and Mn oxides can remove other heavy metals from solution, thus making them less bioavailable (Fan & Wang, 2001). One way they do this is by precipitating heavy metals from solution during oxide formation (Simpson, Rosner & Ellis, 2000); another is direct adsorption onto preformed oxides (Dong, Nelson, Lion, Shuler & Ghiorse, 2000). Sulfide is known to interact with Fe under anaerobic conditions to form a solid, iron sulfide (FeS). Other heavy metals such as copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn) can be removed from solution by displacing Fe and binding to the sulfide. This process has led to a relatively new parameter for evaluating sediment toxicity: simultaneous extracted metal minus acid volatile sulfide (SEM–AVS). The term AVS represents the amount of sulfide in sediments available for binding heavy metals; SEM represents the amount of heavy metals in sediment that could be available to plants and animals. If SEM exceeds AVS, the sediments are potentially toxic (Di Toro et al., 1990; Hansen et al., 1996).
Testing sediments for toxicity generally relies on the use of test organisms. A common test organism is the aquatic larva of the chironomid Chironomus riparius (midge fly). Chironomid larvae, such as those of Chironomus riparius and Chironomus tentans, are detritivores, and they live in intimate contact with sediments. Standardized procedures have been developed that use reduced survival and weight in chironomids as indicators of toxicity in sediments (American Society for Testing and Materials [ASTM], 1992; U.S. Environmental Protection Agency, 1994). Chironomids have also been used in laboratory (Call et al., 1999) and field studies (Liber et al., 1996) to evaluate the influence of parameters such as SEM–AVS and TOC on sediment toxicity. In addition to being valuable test organisms, chironomids are environmentally important components of aquatic food webs (Armitage, Cranston & Pinder, 1995). Several species of chironomids live in Kearny Marsh (Bentivegna, personal observation).
The goals of this study were to evaluate the toxicity of Kearny Marsh sediments and investigate what sediment parameters might be associated with that toxicity. We evaluated sediment toxicity by measuring the ten-day survival and growth of chironomids. Testing was performed with either whole sediment or the detrital fraction (partially decayed organic matter) of the sediment in order to determine what contribution plant matter was making to overall sediment toxicity. Other parameters tested included grain size, TOC, SEM–AVS, and total heavy metal concentrations in sediment and detritus. Our focus was on heavy metals because previous studies had already indicated toxic levels of metals in Kearny Marsh sediments, while the same studies had shown organic contaminants (polychlorinated biphenyls, chlorinated pesticides, and polynuclear aromatic hydrocarbons) to be at lower levels (Langan Engineering and Environmental Services, Inc., 1999). We anticipated that our results would indicate whether there was a need for sediment remediation in the marsh, and if so, what the best remediation approach would be.
Materials and Methods
Site Description
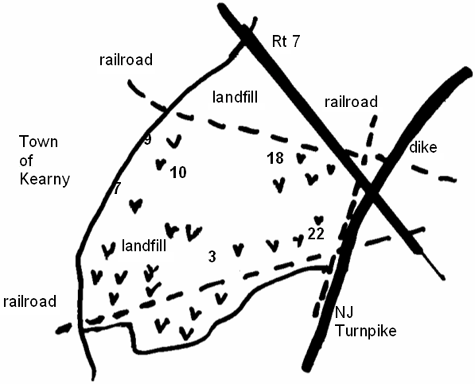
Kearny Marsh is part of the Hackensack Meadowlands in northeastern New Jersey. The marsh is located just west of New York City, New York, and north of Newark, New Jersey. To the west of the marsh is the town of Kearny; to the east are the Hackensack River and its associated wetlands. The marsh is surrounded by highways and railroad tracks that serve commuter traffic in one of the most populous metropolitan areas in the United States (Figure 1).
Water-quality data collected during our study (2002–03) showed the marsh to be an oligohaline wetland, with salinity ranging from 0.5 to 2.6 parts per thousand (ppt). Our study area was shallow, with depths of 0.5 to 3 feet, and had low dissolved oxygen ranging from 0.1 to 3.5 parts per million (ppm) during the months of May through October. Water temperature during the same months ranged from 14°C to 34°C (57.2°F to 93.2°F).
Sediment and Detritus Collection and Analyses
We collected sediment and detritus from six sites in Kearny Marsh. These sampling sites were numbered 3, 7, 9, 10, 18, and 22 (see Figure 1). Substrates were collected on June 5, 2002, and October 18, 2002, using an Ekman dredge. Toxicity testing was done on whole sediment or detritus. We separated detritus from whole sediment on site by sieving the sediment through a 1,000-micrometer mesh, using site water. Substrates were stored in polypropylene containers at 4∫C (39.2°F). Sediment was used in toxicity tests within two weeks of collection; detritus was tested within one month of collection. We analyzed sediment and detritus for heavy metal content (described below), as well as for %TOC, grain size, and AVS–SEM. Samples for AVS–SEM were stored at –70°C (–94°F).
Sediment characterization was done as follows. We analyzed TOC and grain size using American Society for Testing and Materials methods (ASTM, 1992). For TOC, we measured samples by the volatile solids technique, which involved drying sediments and burning off organic matter in a furnace for 16 hours at 550°C (932°F). Percentage TOC was calculated based on the change in sediment weight before and after ignition (ASTM method D2974). We determined grain size by drying whole sediments, grinding them up, and then sieving them through different-size meshes to establish percentages of gravel, sand, and silt+clay (ASTM method D422).
We analyzed AVS and SEM according to Allen, Fu, Boothman, Di Toro, and Mahony (1994). We constructed a closed AVS apparatus consisting of an 8- to 16-vessel train linked together with Nalgene tubing. Nitrogen gas was used to volatilize and transport reactants through the train. Each station of the train consisted of one reaction vessel containing oven-dried sediment (7–14 g*), deionized water (200 ml), and hydrochloric acid (HCl) (10 ml of a 6 M solution) to acidify samples; one vessel containing a pH 4 buffer (potassium phosphate 0.05 M) through which gas flowed to acidify the train; and two silver nitrate traps (200 ml 0.1M AgNO3) into which sulfides flowed from the reaction vessel. At the end of the train was 1 M HCl (200 ml) for acidification of sediment samples. Before being passed through the reaction vessels, the nitrogen gas was deoxygenated and acidified by passing it through an oxygen scrubber (0.02 M H4NO3V, 0.014 M HgCl2) and a pH 4 buffer. All solutions in the train were deoxygenated before use. Reactions ran for two hours, after which vessel contents settled for 30 minutes. Sediment sulfide content (AVS) was analyzed by filtering the combined contents of the two silver nitrate traps through 1.2-millimeter filter paper (Fisher Scientific, Pittsburgh), drying the residue for 40 minutes at 104°C (219.2°F), desiccating it for 20 minutes at room temperature), and then determining the change in filter-paper weight. We analyzed the SEM by collecting 100 to 160 milliliters of the acidified water from the reaction vessel and measuring Cd, Cu, Ni, Pb, and Zn, as described below. Silver nitrate traps were standardized by adding 3 to 6 milliliters of 0.1 M sodium sulfide (NaS) to the AgNO3 solution used in the train; then the AVS was analyzed as described above. The AVS from the samples was based on the micromoles (µmol) of sulfides in the traps adjusted for the standard and divided by the quantity of dried sediment added to the reaction vessel, giving µmol/g. The SEM was based on the combined µmol of Cd, Cu, Ni, Pb, and Zn divided by the quantity of dried sediment added to the reaction vessel.
Heavy Metal Analysis
For metal analysis, sediment samples were oven dried (yielding 1–2 g dry weight), weighed, and mineralized in 10 milliliters of trace-metal-grade nitric acid (HNO3) using Teflon bombs in a microwave digester. The resulting mineralized solution was boiled off to near dryness and restored to 10 milliliters volume with 1% HNO3. We analyzed SEM samples without further processing. Cadmium (Cd), chromium (Cr), Cu, Fe, Mn, Ni, Pb, and Zn were analyzed by flame or graphite-furnace atomic absorption spectrophotometry (using Varian Spectra AA-220FS), depending on the metal concentration. Mercury (Hg) analyses were performed by cold-vapor generation (VGA-77) using a Bacharach MAS-50D mercury analyzer. Trace Metal Standard 1 (Baker Instra-Analyzed Reagent, lot number V47419) was used as a quality control sample and run with each set of samples along with a blank. Data provided were the average of two replicates.
Toxicity Testing
Subchronic toxicity tests followed standard methods (ASTM, 1992). We used second instar Chironomus riparius and ran the tests for ten days. Chironomids were obtained from a laboratory culture maintained by Bentivegna. Two separate subchronic tests were performed and replicated three times (for a total of six tests) on sediment and detritus collected from all sites in June and October. Each replicate started with ten chironomids, which were not fed for the duration of the experiment. Toxicity measurements were based on chironomid survival and weight. Survival was defined as the number of living individuals found after ten days. We determined chironomid weight by collecting survivors from a particular replicate, blotting them dry, and weighing them together. Initial weights were taken from a representative group of ten chironomids at the start of each experiment for comparison.
Conditions for the toxicity tests were as follows. We put 50 milliliters of sediment or 5 grams of detritus, along with 250 milliliters of test water, in one-liter polypropylene containers. The test water was particle and carbon filtered using CDPRM1206 and CDFC01204 filters (from the Millipore Corporation, Billerica, Massachusetts). Test-water hardness (i.e., its concentration of calcium and magnesium) was 130 mg/L. Substrate and water were combined and allowed to sit overnight; chironomids were added the next day. The containers were kept static, and any evaporated water was replaced daily with distilled or deionized water. The containers were exposed to 12 hours of light and 12 hours of darkness. Temperature ranged from 23°C to 26°C (73.4°F to 78.8°F). The pH was taken at the beginning and end of each experiment (using Sentron Model 2001 pH System, Sentron Inc., Gig Harbor, Washington); pH values ranged from 7.1 to 7.7 for sediments and 7.3 to 7.8 for detritus.
Controls for sediment and detritus toxicity testing were set up as follows. In the sediment tests, both positive and negative controls consisted of chironomids exposed to acid-washed sand (from American Stone-Mix, Inc., Towson, Maryland) in 250 milliliters of test water, and fed biweekly on ground fish food (three drops of 0.1 g/ml Tetracichlid; Tetra GMBH, Melle, Germany). Cadmium (Cd) was added to the positive control to a concentration of 0.3mM, while the negative control received no Cd. We fed the control chironomids because the sand had no nutritional value and would not support chironomid survival or growth over a ten-day period. Positive and negative controls for the detritus tests consisted of detritus collected from site 3, along with the same amounts of test water and fish food used in the sediment controls. The positive detritus control also had Cd added to a concentration of 0.3 M, while the negative control had no added Cd. A positive control was not run for June detritus. We fed the detrital control chironomids in order to provide them with an alternative, uncontaminated source of food and distinguish their responses from those of the test chironomids that were exposed to site 3 detritus alone. The pH for the controls ranged from 7.3 to 7.9 in the sediment tests and 6.8 to 7.4 in the detritus tests.
Statistical Analyses
We performed statistical analyses by combining data from the two experiments run for each substrate (sediment and detritus) at each collection time (June and October). Statistical differences for survival and growth in the sediment and detritus treatments were determined by one-way ANOVA, followed by Tukey post hoc tests, p ≤ 0.05. Differences between detritus and sediment from each site and collection time were determined by independent sample T-test, p ≤ 0.05. The statistical relationship between chironomid survival and growth and sediment parameters was determined by bivariate correlation using the Pearson coefficient in a two-tailed test, p ≤ 0.05. All statistical analyses were performed using SPSS software (Version 12.0).
Results
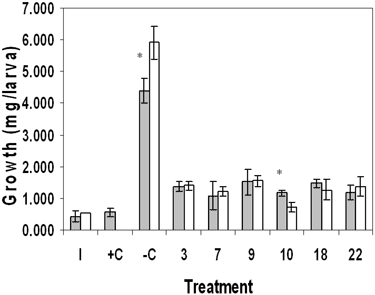
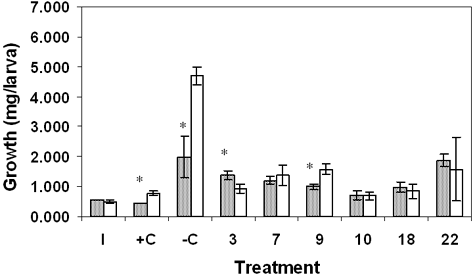
In order to investigate the role of sediment versus detritus toxicity of marsh sediments, we tested whole sediment and its detrital fraction separately. Results for the sediment toxicity tests showed significant differences in survival between sites (Table 1). In June, survival for site 7 was significantly reduced compared with the negative control (–C) and sites 3 and 22, p ≤ 0.05. Survival in October sediment was significantly reduced in sites 7 and 22 compared with –C and sites 3, 9, 10, and 18. Results for growth also showed statistically significant differences in June and October. For June, site-7 growth was similar to the positive control (+C) growth and significantly reduced compared with growth at sites –C, 9, and 18. October results differed from June in that growth was significantly reduced in sites 9, 10, and 18 compared with –C and site 22. Results for detritus showed no statistically significant effects on survival in June or October, although site-10 survival was suppressed in both months (Table 2). Growth in site-10 detritus was significantly reduced compared with –C and sites 7 and 9 in June and October; site-10 results were similar to +C. In addition, site-18 growth was significantly reduced in October detritus.
Our toxicity test results were complicated by the difficulty of finding all the surviving chironomids due to their small size. The nutritional value of the sediment and detritus in general was poor; unfed larvae only doubled or tripled their weights. Data did show that sediment and detritus from some sites, most consistently 7 and 10, were toxic, as growth was similar to that of +C (Tables 1 and 2). Comparison of the two substrates showed that neither consistently supported growth and survival better than the other (Figures 2 and 3). However, detritus did prove superior to sand in –C.
The results of our sediment characterization tests are presented in Table 3. The TOC levels in sediments were very high, ranging from 7% to 87%. This indicated a large amount of detritus in the sediments, which was expected because of the annual dieback of wetland grasses and poor microbial degradation found in suboxic marshes. Sediments were primarily composed of sand, which ranged from 70% to 94%. When combined, the smaller particles of silt and clay ranged from 4% to 31%. Taken together, the percentage of silt+clay was similar between June and October sediments. There were two notable exceptions: At site 18, June sediments were 2.5 times higher than October sediments, and at site 22, June sediments were 6.3 times higher than those in October. This may have been due to variation in the sediment composition at our sampling sites. The SEM–AVS values were all negative, indicating that more sulfide was present than biologically available metals. The AVS values did show apparent seasonal differences: Values in October were considerably higher than in June for most samples. For example, AVS for site 3 was 385.62 µmol/g in October and 37.37 µmol/g in June.
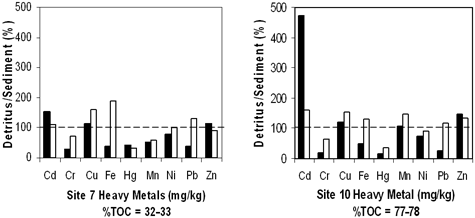
We measured heavy metals in both sediment and detritus and then compared our results to sediment quality guidelines established by the Ontario Ministry of the Environment (1993). These guidelines provide concentrations of metals that have no effect on the majority of sediment-dwelling organisms, designated as "lowest effect level" (LEL), and concentrations that indicate polluted sediment and are likely to affect organism health, designated as "severe effect level" (SEL). Most sites had sediment concentrations of Cr, Cu, and Pb above the SEL (Table 4). Therefore, based on their heavy metal content, sediments should have been toxic. Sites 7 and 9 had the most heavy metals exceeding SEL. Site 22 had no heavy metal concentrations exceeding SEL, but Cd, Cu, Ni, and Pb exceeded LEL. Cadmium did not exceed SEL in any of the sediments but did exceed the LEL for all sites. Results for detritus showed that it was also highly contaminated (Table 5): Copper exceeded SEL in all samples; Cd exceeded SEL for all June samples and site 18 for October. Based on heavy metal concentrations, site 7 was the most contaminated and site 22 was the least contaminated. Substrate comparisons showed that detritus consistently had similar or greater concentrations of Cd, Cu, Ni, and Zn than whole sediment (Figure 4). October detritus also had greater concentrations of Fe and Pb compared with that of sediment. June detritus from site 10 had ten times more Cd than that of sediment. Clearly detritus was an important source of heavy metal contamination in marsh sediments.
We correlated sediment and detritus parameters with chironomid survival and growth in order to ascertain which parameters were having the most effect on toxicity (Table 6). Sediment toxicity was compared with silt+clay, %TOC, and total heavy metal concentrations in detritus (DT-MT) and sediment (SD-MT). Total heavy metal concentrations were the sum of Cd, Cr, Cu, Hg, Ni, Pb, and Zn. We did not include Fe and Mn because we did not consider them toxic at the levels found in this study. For June sediments, the only statistically significant correlation was for survival and DT-MT (–0.865), in that better survival correlated with low heavy metal concentrations in detritus. For October sediments, there were no significant correlations with survival. However, %TOC (–0.863) and DT-MT (–0.939) showed significant negative correlations with growth, indicating that the organic component of the sediments was toxic.
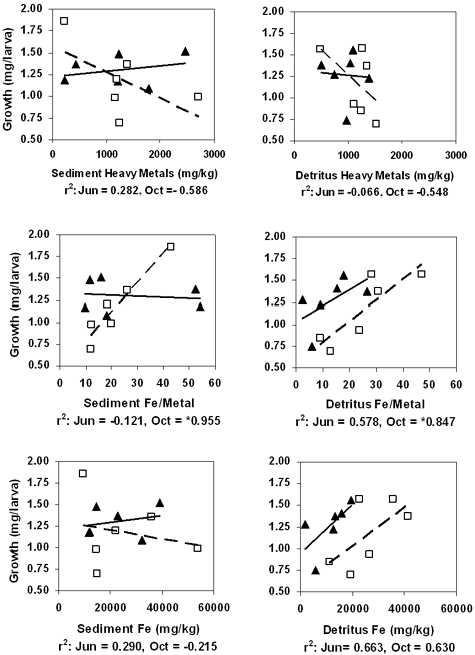
The influence of Fe was evaluated by correlating total metal concentration, AVS SEM, Fe/MT (the ratio of iron to heavy metal), and Fe in sediment and detritus with chironomid survival and growth. The results for total metals, Fe/MT, and Fe are illustrated in Figure 5. June and October sediments showed no statistically significant correlations between Fe parameters and survival. However, there were relatively strong negative correlations for June survival with total metals (–0.719) and Fe (–0.791), which suggested toxicity due to high metal concentrations in general. We found strong but not significant negative correlations for sediment growth with SEM–AVS in June (–0.649) and October (–0.863), indicating sulfides might be limiting heavy metal toxicity. Data for site 22 were not included in the correlations. This site had unusual concentrations of insoluble iron, presumably because it was located close to an old railroad track. The strongest and most significant correlation was between chironomid growth and Fe/MT (0.955) in October sediments. This indicated that sediments with a high proportion of Fe were less toxic. Total metals in detritus correlated poorly with chironomid survival and growth in June (0.392 and –0.060, respectively) and October (0.076 and –0.392, respectively). Correlations improved when Fe content was considered. There were nonsignificant positive relationships between growth and Fe/MT and between growth and Fe in both June (0.575 and 0.663, respectively) and October (0.873 and 0.676, respectively) detritus. Correlations were statistically significant for survival and Fe (0.900) in June detritus and for growth and Fe/MT (0.873) in October detritus. Overall, our data indicates that metals in detritus are an important source of sediment toxicity and that Fe in detritus supports better growth and survival.
Discussion
We characterized Kearny Marsh sediments in terms of common parameters such as grain size, %TOC, and SEM–AVS in order to investigate to what extent they might be contributing to, or moderating, sediment toxicity. The TOC in Kearny Marsh sediments ranged from 7% to 87% and was greater than 32% in most samples. This large amount of organic matter, found primarily in the form of poorly decomposed plant matter, was probably due to suboxic conditions in the marsh. Similar TOC levels (50%–70%) have been found in oligohaline wetlands (approximately 0.5 ppt–2 ppt salinity) in Canada (Bendell-Young, Thomas & Stecko, 2002). The TOC has varied widely, even for similar ecosystems. For example, TOC in Foundry Cove, an oligohaline wetland in the Hudson River watershed, New York, was found to be 0.8% to 13% (Hansen et al., 1996), much lower than that of Kearny Marsh. Kearny Marsh grain size was dominated by sand, typically at levels greater than 80%. These sand levels were similar to those found in Massachusetts salt marshes, which averaged 80% (Hansen et al., 1996). Since Kearny Marsh was once connected to the Hackensack River estuary system, the high percentage of sand in its substrate seems reasonable. The AVS values in sediments were high: 5 to 79 µmol/g in June and 45 to 499 µmol/g in October. These AVS levels were comparable with those found in other suboxic wetlands, which ranged from 50 to 400 µmol/g (Sundelin & Eriksson, 2001). The AVS concentrations well exceeded SEM in most samples, suggesting that sediments should not be toxic. This indicates that some other factor(s) caused the poor growth of chironomids in the toxicity tests (see below).
When testing sediments for toxicity, researchers commonly use test organisms that incorporate and respond to multiple toxicity parameters and allow them to discriminate measurable concentrations of contaminants. The bulk of the literature on the subject shows that various, natural sediment components can interact with contaminants and limit their bioavailability and associated toxicity (Ankley et al., 1996). In this study, we tested whole sediment and its detrital fraction from several sites in the Kearny Marsh using larvae of a common benthic macroinvertebrate, Chironomus riparius (midge fly). Our data showed that neither whole sediment nor detritus supported good chironomid growth (Tables 1 and 2). Chironomids merely doubled or tripled their size over a ten-day exposure period while controls fed on an alternate food source usually grew to ten times their initial weights. Since chironomid growth correlated with several factors known to control heavy metal bioavailability (Table 6), it is likely that effects were due to sediment and detritus toxicity. The case for poor sediment quality was supported by the absence of resident organisms; only an occasional nematode was actually found in sediments. Ingestion appeared to be an important route of contaminant exposure for the larvae, as there was an excess of free sulfides (AVS) to bind up any free heavy metals that might be absorbed through larval cuticles or gills. Also, larvae grew well in sediment and detritus samples to which fish food was added (all data not shown). For example, larvae in site 3 detritus showed good mean growth (4.698 ± 0.302) with fish food but poor mean growth (0.928 ± 0.146) without it.
Previous studies have shown that the major contaminants in Kearny Marsh sediments were heavy metals (Langan Engineering and Environmental Services, Inc., 1999). In this study, five of six sites had sediments with SELs of Cr, Cu, and Pb based on established sediment quality guidelines (Table 3). Two sites, 7 and 9, were also severely contaminated with Hg. Site 9 had the highest level of total toxic metals. Metal concentrations were similar to or greater than those found in the Hackensack River and Newark Bay, which were 10±6 mg/kg Cd, 237±222 mg/kg Cu, 2.1±2.6 mg/kg Hg, 39±49 mg/kg Ni, 421±571 mg/kg Pb, and 395±403 mg/kg Zn (Bonnevie, Huntley, Found & Wenning, 1994). Surprisingly, detritus not only had heavy metal concentrations above sediment LELs, but it also contained higher concentrations of Cd, Cu, Pb, and Zn than sediments (Table 4 and Figure 4). Kearny Marsh is dominated by the wetland grass Phragmites australis (common reed), which actively accumulates heavy metals in its roots (Peltier et al., 2003). Decomposition of these contaminated roots over time could have contributed to the heavy metals in the marsh detritus. Alternatively, the detritus could have adsorbed the heavy metals from overlaying water. Windham and coworkers found that submerged litter from wetland plants accumulated heavy metals in excess of sediment concentrations (Windham, Weis & Weis, 2004). As in our study, they determined that Cu, Pb, and Zn adsorption was greater than that of Cr and Hg. In either case, detritus might have contributed to an unstable pool of metals that were more or less available during the year. This was supported by metal concentrations in detritus that were consistent with the release of Cd under oxic conditions (early June) and of Pb under suboxic ones (early fall) (Reddy & Patrick, 1977).
Several sediment and detrital parameters showed statistically significant correlations with chironomid survival and growth (Table 6). This indicates that the parameters were influencing toxicity even though responses between sites did not appear to be very different (Figures 2 and 3). In our sediment experiments, metal concentrations in detritus (DT-MT) correlated with chironomid survival in June samples (–0.874) and chironomid growth (–0.940) in October samples. Correlations indicate that chironomid survival and growth were better when metal concentrations in detritus were low, and that metal concentrations in whole sediment were less influential. The complexity of larval responses was shown by the lack of correlation between DT-MT and chironomid growth in June (–0.174). In this instance, it is possible that the death of some larvae allowed less sensitive ones to acquire more food and grow normally. Iron concentrations in sediment and detritus appeared to be an important factor controlling substrate toxicity. When Fe levels increased or exceeded relative to the combined total of other metals (Fe/MT), toxicity was reduced. This relationship was seen in October sediment and detritus and in June detritus (Figure 5). Iron chemistry of sediments is known to control heavy metal bioavailability. Research has shown that Fe oxide precipitates can adsorb heavy metals (Dong et al., 2000) and that sulfides can exchange Fe for other toxic metals, forming less available metal sulfide precipitates (Hansen et al., 1996). The formation of metal sulfides has been used to explain the apparent lack of toxicity for anaerobic sediments that are highly contaminated with heavy metals (Lau & Chu, 2000). In our studies, sulfides did not appear to be a significant factor as measured by SEM–AVS. The AVS values did show seasonal variation, being lower in June and higher in October (Table 3). Research has shown that wetland plants create oxygenated microenvironments around their roots and thereby release sulfides from sediments (Azzoni, Giordani, Bartoli, Welsh & Viaroli, 2001). Data presented here support the idea of oxygenated microenvironments, in that there were fewer sulfides in sediment during active plant growth (June) and more when plants are less active (October). However, sediments were toxic even when there were high levels of sulfides (AVS) available to bind heavy metals. Correlations with detrital parameters indicate that organic Fe complexes are more important for moderating the toxic effect of heavy metals.
The results of this project show Fe and detritus to be controlling factors of toxicity at Kearny Marsh. These findings suggest several approaches for remediating Kearny Marsh. The marsh is suboxic, which is the primary factor limiting biodiversity. Increasing water circulation by reconnecting it to the Hackensack estuary and/or increasing coverage by wetlands plants would likely improve dissolved oxygen levels. One concern about increased circulation would be the release of heavy metals from metal sulfides and detritus into the water column and the subsequent contamination of the Hackensack estuary. However, the substantial amount of Fe in marsh sediments and the production of metal-binding Fe oxides under more aerobic conditions would probably limit the redistribution of toxic heavy metals (Liang, McNabb, Paulk, Gu & McCarthy, 1993). Another approach would be to cap sediments. In aquatic ecosystems this is usually achieved with sand. However, sand does not provide an appropriate substrate for the type of macroinvertebrates found in oligohaline wetlands. Capping with clay-based substrates amended with Fe and organic matter would complement the natural chemistry of the area and provide a better substrate for macroinvertebrate colonization. We recommend further studies of metals in the sediment of Kearny Marsh and also an investigation of the potential contribution of organic contaminants to sediment toxicity.
Acknowledgments
This work was supported by a Meadowlands Environmental Research Grant (MERI) to Carolyn Bentivegna, 2002–03. Paul Dombrowski provided technical support for AVS analyses. Reena Patel assisted with toxicity testing. Sediment collection and analyses were supported and/or performed by MERI personnel including Kirk Barrett, Edward Konsevick, Yefim Levinsky (metals), Mary Neer (TOC-grain size), and Joseph Sarnoski (substrate collection and environmental monitoring).