Biodiversity Patterns and Conservation in the Hackensack Meadowlands, New Jersey
by Erik Kiviat and Kristi MacDonald
¹Hudsonia Ltd., P.O. Box 5000, Annandale, NY 12504
²Ecology and Evolution Program, Rutgers University, 1 College Farm Road, New Brunswick, NJ 08901
Abstract
The 8,300 hectares (roughly 20,500 acres) of wetlands, uplands, and developed areas of the Hackensack Meadowlands in northeastern New Jersey are a major urban biodiversity reservoir in the New York metropolitan region. Species documented so far include 260-plus birds (33 of which are state-listed as endangered, threatened, or declining), 22 mammals, 51-plus fishes, 51 bees, and 420 plants. Wetlands make up 3,200 hectares (roughly 7,800 acres) of the Meadowlands, and they include brackish and freshwater marshes dominated by the common reed (Phragmites australis) as well as cordgrass (Spartina) marshes and hardwood swamps. Upland habitats are found on bedrock hills and wetland fill. The mix of wetlands and uplands gives rise to a diversity of plant and animal life. The marshes and swamps of the Meadowlands provide critical habitat for many species, and several species also rely on the upland habitat types. Relatively well-studied groups, such as birds and fishes, have received the most attention from local conservation planners. However, other, poorly studied organisms (invertebrates, for example) also contribute to the biodiversity value of the Meadowlands and should be taken into account. Conservation planners should also consider the constraints and opportunities imposed by the urban context of the Meadowlands, especially with regard to the management of habitats dominated by Phragmites. Factors associated with urbanization, such as sediment contamination, as well as the presence of many common and rare species in reed marshes, indicate that alteration rather than eradication of reed stands should be considered. In addition to a continued focus on wetlands, successful maintenance and enhancement of biodiversity in the Meadowlands will require attention to upland habitats, including some that are artificial. Principles of biodiversity conservation in the Meadowlands are broadly applicable to large urban wetlands elsewhere.
Keywords: Biodiversity; degraded wetlands; habitat management; Hackensack Meadowlands, New Jersey; Phragmites; urban wildlife; wetland restoration
Introduction
With nearly all the 50 largest metropolitan areas in the United States located on coasts or major waterways, urban expansion has unavoidably influenced, and been influenced by, wetlands. Because urban wetlands are intensely altered by human activities, they have often been accorded lower priority for protection by regulators and environmentalists. However, growing recognition of the important functions of urban wetlands in densely settled regions, including water filtration, flood control, and green space, has begun to reverse this attitude. Recent research has demonstrated that urban wetlands have unique ecological and social values precisely because they are located within an urban context (Ehrenfeld, 2000).
Other research has demonstrated that species diversity in urban habitats is low compared with more rural or pristine habitats due to the dominance of a few hardy generalist or invasive species and a loss of sensitive, specialist species (Adams, 1994). Because diversity of species is an important measure of the value of wetlands, this has often led to the destruction of natural wetlands in urban areas. We argue that, to the contrary, some urban wetlands support a high richness and abundance of fauna and flora, and that this diversity of species is influenced by the urban context in both positive and negative ways. Furthermore, species richness depends on the taxa studied and the adequacy of survey techniques in detecting rare species. The importance of common species increases in urban areas, where many species common in rural or wildland areas do not survive, and where wildlife is available for viewing by large numbers of people. We believe a different framework is needed for evaluating biodiversity in urban wetlands. We therefore present a case study of the Hackensack Meadowlands, which contain 3,200 hectares (7,907 acres) of wetlands just five kilometers* from midtown Manhattan.
Many important decisions are being made about landscape preservation, habitat management and restoration, remediation of contamination, and development in the Meadowlands. For example, there are plans to preserve as a wildlife refuge and environmental park a total of more than 3,000 hectares (7,413 acres) of wetlands, and approximately 1,400 hectares (3,460 acres) have already been preserved (Kiviat & MacDonald, submitted for publication). There is an ongoing project to cap and develop 531 hectares (1,312 acres) of inactive solid waste landfills for golf courses and associated facilities ("Landfills to Open Space," 2003). The largest remaining privately owned wetland in the Meadowlands, the 236-hectare Empire Tract, is about to be preserved, and decisions on how to manage this site will need to be made. A comprehensive restoration plan for the Meadowlands is being prepared. Although considerable funds have been expended on biological studies, most taxa, biodiversity patterns, and ecological processes have been considered barely or not at all in the planning of land use and restoration.
In this paper, we analyze biodiversity patterns, identify the significance of the Meadowlands for biological conservation, and discuss implications for land-use planning and habitat management. This discussion is intended to broaden the framework for decision making in the Meadowlands and other areas of the New York–New Jersey metropolitan region. As urbanization rapidly proceeds, areas like the Meadowlands are increasingly important for conservation and offer a glimpse of the environmental future of many now rural and wildland areas.
Methods
Study Area
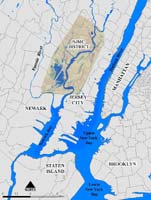
Figure 1. Map showing the location of the official Hackensack Meadowlands District (green) in the New York–New Jersey Harbor estuary region. Contiguous wetlands and riparian areas exist both north and south of the district and should be considered as part of the Meadowlands biodiversity landscape. (Map courtesy of New Jersey Meadowlands Commission.)
The Hackensack Meadowlands proper are about 16 kilometers long north to south and cover an area of about 8,300 hectares (roughly 20,500) that was once almost entirely wetland (see Quinn, 1997; Day, Staples, Russell, Nieminen & Milliken, 1999). The Hackensack Meadowlands District consists of 7,889 hectares (19,494 acres) of residential, commercial, and industrial development; landfills; roads and railways; natural uplands; and wetlands (Figure 1). In this paper, we also consider adjoining wetlands and floodplains, including the narrow riparian area extending north from Teterboro along the upper Hackensack River estuary. The Meadowlands are shown on U.S. Geological Survey 7.5-minute topographic map quadrangles (Elizabeth N.J.–N.Y. 1995, Hackensack N.J. 1997, Jersey City N.J.–N.Y. 1967 [Photorevised 1981], Orange N.J. 1955 [Photorevised 1970], Weehawken N.J.–N.Y. 1967, Yonkers N.J.–N.Y. 1956).
Bedrock underlying the Meadowlands is shale, sandstone, and, locally, diabase and hornfels (Wolfe, 1977). The elevation of the wetlands is 0 to about 3 meters above sea level; bedrock hills and landfills rise to as much as 30 to 50 meters. Thirty-meter clay bluffs and 10-meter cliffs of shale and sandstone occur locally at the edges of the Meadowlands (Bosakowski, 1983; Kiviat, personal observation). Deep mineral and organic wetland soils are found in most of the Meadowlands, and in limited areas there are natural upland soils, most of which have been highly altered.
Meadowlands habitats include the deep tidal channel of the Hackensack River main stem; a variety of brackish tidal creeks, canals, and ditches; tidal marshes ranging from nearly fresh to very brackish; impounded brackish and nearly fresh marshes with little or no tidal flux; nontidal marshes and hardwood swamps; woodlands, shrublands, and meadows on low-lying wetland fill or elevated solid waste landfills; meadows, scrub, and woodland on clayey or sandy soils; road verges, dikes and berms, industrial areas, residential yards, urban parks, and other developed areas; storm-water ponds and clay pit lakes; and clay bluffs and bedrock outcrops that vary from quarried to nearly undisturbed (Figures 2 to 8; Kiviat & MacDonald, 2002). Only stumps remain from once extensive Atlantic white cedar (Chamaecyparis thyoides) swamps (Heusser, 1963; Harmon & Tedrow, 1969).
Salinity in the Hackensack River ranges from 0 to about 24 parts per thousand (ppt) (C. Woolcott, personal communication, 2004). Salinity is generally highest in late summer and fall and lowest in spring (Kraus & Bragin, 1988). Tidal circulation has been modified by the Oradell Dam at the upper end of the estuary and by ditches, dikes, tide gates, dams, road beds, fill, and subsequent breaching of a few water-control structures. These structures drained freshwater from many areas, impounded other areas, or prevented brackish water intrusion.
Until the late 1960s, most of the sewage discharged into the Hackensack River was untreated, according to a study by the Interstate Sanitation Commission (Crawford, Bonnevie, Gillis & Wenning, 1994). There are now 7 sewage treatment plants, 32 combined sewer overflows and 12 emergency overflows in the Meadowlands District (Day et al., 1999). The annual range of dissolved oxygen is 1.0 to 15.5 milligrams per liter (Day et al., 1999) in the Hackensack River. Lead, mercury, zinc, chromium, PCBs, PAHs, petroleum hydrocarbons, and DDT metabolites contaminate the soils, submerged sediments, water column, and aquatic life of the Meadowlands, in some places reaching levels considered hazardous under federal regulatory standards (Bonnevie, Wenning, Huntley & Bedbury, 1993; Crawford et al., 1994; Huntley, Bonnevie, Wenning & Bedbury, 1993; Huntley, Bonnevie & Wenning, 1995; Hackensack Meadowlands Development Commission [HDMC], 1997, 2002; Durell & Lizotte, 1998).
Data Sources
Much biological fieldwork has been conducted in the Meadowlands, but few results have been published in the formal literature. This paper is based on data from formal scientific literature, gray literature (e.g., agency reports and consulting reports), master's and Ph.D. theses, a few popular articles, unpublished data, and discussions with scientists and naturalists (see Kiviat & MacDonald, 2002). We also conducted reconnaissance fieldwork in a number of areas of the Meadowlands from 1999 to 2004. In most cases there has been no analysis of how the Meadowlands support biodiversity. We set the stage for more formal analyses by assessing the state of knowledge about species diversity and the underlying ecological processes that support it. We also discuss opportunities to maintain or enhance Meadowlands biodiversity.
Results
Plant and Animal Life
Despite decades of study in the Meadowlands, data on the distribution and abundance of organisms are mostly qualitative and narrow in taxonomic representation. Birds are moderately well studied at the site level, and the fishes and macrobenthos have been sampled in the larger waterways and a few smaller tributaries. Most other taxa have been studied little or not at all. Table 1 summarizes what is known about various types of organisms in the Meadowlands. Here we discuss selected taxa of conservation interest or other importance.
Mammals
Of about 45 mammal species occurring in northeastern New Jersey (Whitaker & Hamilton, 1998), 22 have been reported from the Meadowlands, including 4 introduced species (dog, cat, Norway rat, and house mouse). Only 2 of 6 possible species of shrews and moles, 1 of 7 bats, and 8 of 16 rodents have been reported, indicating that there should be further fieldwork on the smallest mammals. Most reported Meadowlands mammals are common, urban-tolerant species of wetland or upland habitats (Kiviat & MacDonald, 2002), with the possible exceptions of the masked shrew (Sorex cinereus), eastern mole (Scalopus aquaticus), and meadow jumping mouse (Zapus hudsonicus). Two mammals known to be sensitive to environmental contaminants, mink (Mustela vison) and harbor seal (Phoca vitulina), are very rare in the Meadowlands (Kiviat & MacDonald, 2002).
The eastern cottontail (Sylvilagus floridanus), white-footed mouse (Peromyscus leucopus), meadow vole (Microtus pennsylvanicus), common muskrat (Ondatra zibethicus), Norway rat (Rattus norvegicus), and house mouse (Mus musculus) are apparently common and presumably important prey of predatory mammals, birds, and snakes. The Norway rat and house mouse are believed to have declined greatly in recent years due to the closing of all but one of the many garbage landfills. The common muskrat may be considered a keystone species because its feeding and building activities have major effects on vegetation, soils, microtopography, and animal habitats (Kiviat, 1978; Connors, Kiviat, Groffman & Ostfeld, 2000). The muskrat has declined in Hudson River marshes in recent decades (Kiviat, unpublished) and should be monitored in the Meadowlands. The beaver (Castor canadensis) is unknown (A. Galli, personal communication, 2001), and the white-tailed deer (Odocoileus virginianus) is mostly limited to the forests of northern areas (R. Kane, personal communication, 2000). Regional trends suggest that both species are likely to increase, and this would have substantial influences on the Meadowlands environment.
Birds
More than 260 species of birds, 5 of them introduced species, have been reported in the Meadowlands (New Jersey Turnpike Authority [NJTA], 1986; Meadowlands Environment Center, n.d.; Kiviat, personal observation). This species diversity, which includes resident, migrant, breeding, and wintering birds, is supported by the large expanses of estuarine marshes interspersed with diverse freshwater and upland habitats. There are 33 state-listed endangered, threatened, declining, or rare birds in the Meadowlands: 12 hawks and owls, 7 songbirds, 4 herons, 4 Charadriiformes, 2 rallids, and 4 others (a grebe, a cormorant, a hummingbird, and a woodpecker). Twenty of the listed species are generally associated with waters or wetlands, nine with grasslands, and four with upland forests.
The Meadowlands are one of 11 critical migration corridors in New Jersey identified by Dunne, Kane, and Kerlinger (1989). At one site, daily counts of migrant sandpipers have exceeded 5,000 in most years (Day et al., 1999). The Meadowlands are used intensively by wintering, breeding, and migrating waterfowl and have been designated an area of special concern under the North American Waterfowl Management Plan (Day et al., 1999). Midwinter aerial survey counts of waterfowl in the Meadowlands average 2,000 birds per day (Day et al., 1999). In addition, the Meadowlands are an important foraging area for herons from nesting colonies in other areas of the New York–New Jersey Harbor estuary complex (Murray, 1990; Day et al., 1999).
The Meadowlands support some surprising breeding birds. The American woodcock breeds in quaking aspen (Populus tremuloides) patches and other vegetation on garbage landfills (Rawson, 1993; R. Kane, personal communication, 2000; Kiviat, personal observation). Nests of the least tern (Sterna antillarum), listed as endangered in New Jersey, have been found on dredged material deposits and the roof of a commercial building (Day et al., 1999; R. Kane, personal communication, 2001; K. Spendiff, personal communication, 2003). Regionally rare breeding populations of the ruddy duck (Oxyura jamaicensis), which nests solely in common reed (Phragmites australis) marshes in New Jersey (Kane, 2001a), occur at two brackish water impoundments. At least one pair of northern harrier (Circus cyaneus) breeds in the Meadowlands (NJTA, 1986; Kane & Githens, 1997; Day et al., 1999; N. Tsipoura, personal communication, 2003); the statewide breeding population of this species is listed as endangered. Roosting congregations of northern harrier and short-eared owl (Asio flammeus), principally in common reed stands, no longer exist, probably because the closure of most garbage landfills greatly reduced populations of small rodent prey (Bosakowski, 1983, 1986; T. Bosakowski, personal communication, 2004; H. Carola, personal communication, 2004). Upland meadows and patches of woody vegetation, principally on fill, as well as small remnant forests in the north, support large numbers of Neotropical migrant warblers, vireos, kinglets, and flycatchers during spring and fall migrations (Kane & Githens, 1997). Fleshy-fruited shrubs and vines make upland habitats highly attractive to fall migrants (Suthers, Bickal & Rodewald, 2000). Limited areas of swamp or upland forest restrict the potential breeding habitat for many species. The swamp forest at Teterboro Airport has poorly developed shrub and herb layers and low breeding-bird diversity (MacDonald, personal observation). There are few data on upland birds.
Reptiles and Amphibians
Of 15 species of snakes occurring in northeastern New Jersey (Conant & Collins, 1991), 8 have been reported in the Meadowlands. Documentation is scant for three of these, and some may have been extirpated. Contaminants and the limited extent of natural uplands and low-salinity habitats may limit snake diversity. The northern water snake (Nerodia sipedon) and other species may once have been common (Quinn, 1997).
Six of the eight turtle species occurring in northeastern New Jersey have been reported in the Meadowlands (excepting sea turtles; Conant & Collins, 1991; Kiviat & MacDonald, 2002). Documentation is scant for two of the six species. Snapping turtle (Chelydra serpentina) and diamondback terrapin (Malaclemys terrapin) are locally common. Female terrapins attempting to cross, or nest on, major highways such as the New Jersey Turnpike are often killed (Urffer, 2002), and such road mortality may limit turtle populations in general, because they are highly mobile.
Ten of the 26 species of amphibians (13 frogs and 13 salamanders; Conant & Collins, 1991) occurring in northeastern New Jersey have been reported in the Meadowlands. Documentation is scant for six of the ten. A NJTA study (1986) found only two species, green frog (Rana clamitans) and Fowler's toad (Bufo fowleri). No salamander has been reported. The scarcity of natural upland soils and high-quality, fresh surface waters probably accounts for the low species richness of amphibians. Many reptile and amphibian species are intolerant of urbanization (e.g., Schlauch, 1976). One Meadowlands reptile is an introduced species, the red-eared slider (Trachemys scripta elegans); all amphibians are native.
Fishes
The Meadowlands are considered important habitat for migratory fishes (Day et al., 1999), and many migratory species have been found there, but there is little information on spawning and nursery areas. Atlantic tomcod (Microgadus tomcod), formerly listed as threatened in New Jersey, uses the Hackensack River from near its mouth to Sawmill Creek as a nursery, refuge, and spawning area (Kraus & Bragin, 1988). However, the species was very rare during a 2001–2003 resurvey (C. Woolcott, personal communication, 2004). The lower Hackensack River system was declared essential fish habitat by the National Marine Fisheries Service for six species: red hake (Urophycis chuss), black sea bass (Centropristis striata), Atlantic butterfish (Peprilus triacanthus), and three flounders (Pleuronectidae and Bothidae), and designation was pending for bluefish (Pomatomus saltatrix) and Atlantic herring (Clupea harengus) (Day et al., 1999).
Low-salinity tidal marshes in the Hudson River support moderately rich fish communities (Mihocko et al., 2003). In the Meadowlands, Feltes (2003) sampled 13 species during four years in mitigated and nonmitigated portions of Harrier Meadow, whereas the U.S. Army Corps of Engineers (2000) reported only 4 species in small waterways on the Empire Tract. Lower species richness in the Corps of Engineers data may be due to habitat diversity and sampling effort, but also to low dissolved oxygen (DO) and other water quality problems, which reduce species diversity in marsh creeks, small ponds, and even the mainstem of the Hackensack River (e.g., Day et al., 1999). In the Mill Creek system of the Meadowlands, Raichel, Able, and Hartman (2003) found that mummichog (Fundulus heteroclitus), the most abundant Meadowlands fish, was less numerous as larvae in common reed habitat than in smooth cordgrass (Spartina alterniflora) habitat, although the abundance of adults was similar. They identified two potential explanatory factors: the tendency of common reed to fill in irregularities in the marsh surface that are used by fish larvae at lower tide stages, and lower abundance of small animals that constitute potential prey for mummichog larvae. The spotfin killifish (Fundulus luciae) has been reported just downriver of the Meadowlands (Yozzo & Ottman, 2003), suggesting that this species and other uncommon fishes may occur in the Meadowlands.
Invertebrates
There have been few surveys of aquatic and terrestrial invertebrates in the Meadowlands. The NJTA (1986), from an area that included the Meadowlands and extended well to the south, reported the following 50 estuarine and freshwater benthic macroinvertebrates: 3 Oligochaeta, 8 Polychaeta, 1 Hirudinea (leech), 7 Gastropoda (snails), 4 Bivalvia, 15 Crustacea, 1 Tunicata (tunicate), 1 Tentaculata (ctenophore), 1 Nematoda (roundworm), 4 Insecta, 1 Anthozoa, 1 Bryozoa (moss-animal), 1 Cnidaria, 1 Hydrozoa, and 1 Rynchocoela. The same study reported approximately 42 species from the Hackensack River and its major tributaries (Kiviat & MacDonald, 2002). Organisms tolerant of pollution and low DO were dominant (NJTA, 1986). Kraus and Bragin (1988) found 53 species in the Hackensack River and tributaries. Strayer and Smith (2001) reported 218 species from the freshwater-tidal Hudson River, of which 146 were associated with soft sediment. The larger number of species reported from the Hudson compared with the Hackensack may be due to the larger size of the system, more study, and identification to lower taxonomic levels as well as to better environmental quality (however, the Hackensack studies spanned a broader salinity gradient).
The clam shrimp Caenestheriella gynecia was abundant in permanent rain puddles on the dirt surface of a gas pipeline road in the Empire Tract (Kiviat & MacDonald, 2002). This species occurs only at about ten known localities range-wide in the eastern U.S. (R.E. Schmidt, personal communication, 2003).
Butterflies require specific larval food plants, and adults of most species require nectar sources. Many butterflies have narrow habitat affinities, and nonmigratory species may require specific overwintering habitats. Many species, some now rare in New Jersey, were reported a century ago from "Newark" (Gochfeld & Burger, 1997), which probably included the Hackensack Meadowlands as well as the now-filled Newark Meadows. Certain high-quality nectar plants are common in the Meadowlands (e.g., purple loosestrife, Lythrum salicaria, an introduced invasive species that is visited by many species). Although larvae of the broad-winged skipper (Poanes viator) specializes on common reed and is probably very abundant in the Meadowlands, the larvae of many species of skippers and other butterflies feed on wetland grasses and sedges other than common reed and cordgrasses. The Meadowlands lack extensive stands of most other grasslike plants, which may limit butterfly diversity.

Figure 4. Common reed (Phragmites australis)—tree-of-heaven (Ailanthus altissima) stand at Cromakill Creek, Hackensack Meadowlands, New Jersey. The eastern cottontail eats bark and the northern cardinal eats seeds of tree-of-heaven. Common reed supports a variety of mammals, birds, insects, and spiders. Photograph by Erik Kiviat.
There is a general concern about the decline of native pollinators, especially bees, in North America (Shepherd, Buchmann, Vaughan & Black, 2003). A diverse community of mostly native bees was studied at an inactive garbage landfill in the Meadowlands, where there were various nectar plants and nest habitats in eroding soil and hollow plant stems, including those of common reed (Yurlina, 1998; G.R. Robinson, personal communication, 2003). Other types of wetland fill containing nectar plants such as goldenrods (Solidago), the introduced invasive Japanese knotweed (Fallopia japonica), and purple loosestrife are also potentially attractive to bees, butterflies, and other flower visitors.
An area of the Meadowlands was surveyed for lady beetles (Coccinellidae) and their most important prey, aphids (Aphididae) (Angalet, Tropp & Eggert, 1979). The mealy plum aphid (Hyalopterus pruni), which alternates between common reed and woody plants of the genus Prunus, and several aphids found on mugwort (Artemisia vulgaris), an introduced species, were very abundant and the principal prey of lady beetles in the Meadowlands (Angalet et al., 1979). A native and an introduced lady beetle overwintered in association with the tussock-forming redtop grass (Agrostis gigantea), common mullein (Verbascum thapsus), and planted pines (Pinus sylvestris, P. resinosa), all introduced species (Angalet et al., 1979). The 15 species of aphid host plants reported were mostly introduced, weedy species of ruderal habitats or upland meadows.
Human-biting ticks are scarce in the Meadowlands, probably due to the limited occurrence of woodlands and white-tailed deer. Wood tick (Dermacentor variabilis) and black-legged (deer) tick (Ixodes scapularis) occur locally (Kiviat, personal observation).
Vascular Plants
The Meadowlands have a moderately diverse flora (Sipple, 1972). A list from Brooklyn Botanic Garden's New York Metropolitan Flora Project, included in Kiviat and MacDonald (2002), contained 416 species. The Torrey Botanical Society reported 115 and the NJMC reported 145 species from Laurel Hill (Quinn, 1997), a highly altered igneous upland and wetland fill area.
New York City supports a number of rare plant species (e.g., Venezia & Cook, 1991), yet few species considered rare statewide have been reported from the Meadowlands. In Kingsland Creek and upper Penhorn Creek there are large stands of floating marsh-pennywort (Hydrocotyle ranunculoides) (Kiviat, personal observation), which are ranked S1 (the "S" rank, from the New Jersey Natural Heritage Program ranking system, refers to the number of localities where the species has been found in recent years in the state, with S1 the rarest and S5 the most common). A single plant of wafer-ash (Ptelea trifoliate), also ranked S1, was found on Laurel Hill; it is unclear whether this is a natural occurrence (Labriola, 2000).
Several other native plants occurring in the Meadowlands may be rare in northeastern New Jersey. These include five-angled field dodder (Cuscuta pentagona), beardtongue (Penstemon digitalis), starry campion (Silene stellata), Virginia mountain mint (Pycnanthemum virginianum), pale corydalis (Corydalis sempervirens), and post oak (Quercus stellata) (Labriola, 2000; Kiviat, personal observations). Many fen and bog species were once found in the Meadowlands (Sipple, 1972), and some may survive in swamps of the northern Meadowlands, for example, at Teterboro Airport. The Metropolitan Flora list for the Meadowlands includes only four Carex species (sedges). This may reflect degradation of much of the Meadowlands, although additional survey work in the fresh swamps and wet clay meadows of the northern Meadowlands would surely find many additional Carex.
Mosses, Lichens, Terrestrial Algae, and Fungi
Although there has been no survey of Meadowlands "cryptogams" exclusively, diversity appears to be low. Mosses and lichens are rare, local, and mostly limited to small patches. Mosses and lichens do not generally thrive in urban areas due to pollution, low humidity, and acidification of bark due to air pollution (Gill & Bonnett, 1973), and the scarcity of large living and dead tree trunks, natural rock surfaces, natural soils, and other preferred substrates. This may inhibit macrofungi as well (Gill & Bonnett, 1973). There are some large tree trunks, mostly dead wood of Atlantic white cedar, but the majority of them are subject to flooding by brackish water, which presumably limits the diversity of fungi. Because they are sensitive to air pollution and therefore are rare in cities generally, we were surprised to find any lichens in the Meadowlands. The varied mineral composition of the igneous uplands (van Houten, 1969; Facciolla, 1981) is potentially capable of supporting a diverse community of mosses with different tolerances and requirements; however, surveys have yet to be conducted to determine if such a community exists in the Meadowlands. Sperling and Morgan (2003) reported 77 species of bryophytes from specimens collected in the 1970s and 1980s in the 578 hectares (1,428 acres) of two parks in Queens, New York, and Feuerer, Hertel and Deuter (2003) reported 226 species of epiphytic lichens from Munich, Germany, although 102 had not been found in a century. Only 28 species of lichens were reported in nearby Westchester County, New York (Prince, 1978). No taxonomic data are available on microfungi or other soil microorganisms in the Meadowlands.
Discussion
The patterns of biodiversity in the Meadowlands are a result of natural and artificial conditions acting at various spatial scales. In this section we outline some major factors affecting biodiversity and briefly discuss some of the taxa most affected.
Pollutants
Low dissolved oxygen, high turbidity, and high temperatures may lower diversity of fishes and benthic macroinvertebrates. Levels of metals in marsh plants (Kraus, 1988) are potentially toxic to herbivores such as muskrats, and Kraus (1989) showed that metals moved from sediments via chironomid midges to tree swallows in the Meadowlands. Despite at least locally high levels in Meadowlands sediments, studies have found comparatively low levels of metals and PCBs in fishes, turtles, and birds (Galluzzi, 1981; Albers, Sileo & Mulhern, 1986; Santoro & Koepp, 1986; Weis, Weis & Bogden, 1986; C. McIntyre, unpublished data cited in Kiviat & MacDonald, 2002).
It is remarkable that a region as contaminated as the Meadowlands can support the biodiversity observed and that documented contaminant levels are fairly low in fish-eating animals. Possible explanations are 1. Fish-and crustacean-feeding birds such as pied-billed grebe, herons, bitterns, gulls, and terns are consuming mummichogs, fiddler crabs, or other prey that do not accumulate large amounts of contaminants (see Galluzzi, 1981); 2. Studies have missed the sites, prey species, predator species, population classes, or tissues in which contamination is high; or 3. Common reed and other plants are sequestering metals, or reduced conditions (lack of oxygen) in the sediments are immobilizing metals, making them unavailable to organisms higher in the food chain. Yet potential adverse effects of modest levels of mercury on health of fishes (Uryu, Malm, Thornton, Payne & Cleary, 2001) and waterbirds (Odom, 1975) may be relevant to the Meadowlands: Weis, Smith, Zhou, Santiago-Bass, and Weis (2001) reported that mercury-contaminated mummichogs from 15 kilometers south of the Meadowlands were slower to capture prey and escape predators, had more detritus in their diet, and had reduced growth and longevity compared with the same species in cleaner areas. Further study of contaminants in animal tissues is needed to confirm the low levels reported in the Meadowlands, and research is needed on health effects in animals. State Health Advisories (New Jersey Department of Environmental Protection, 2004) concerning PCBs and dioxin warn against any consumption of blue crab or striped bass from the Hackensack River estuary, and recommend American eel (Anguilla rostrata), white catfish (Ameiurus catus), and white perch (Morone americana) be eaten only once per year by the general population and not at all by high-risk individuals.
Sulfur oxides, nitrogen compounds, ozone, metals, fluoride, and other air pollutants have a wide variety of impacts on organisms (Barker & Tingey, 1992). The low diversity of lichens and mosses in the Meadowlands is presumably at least partly due to air pollution.
Invasive Plants
The proliferation of invasive nonnative or native organisms alters the composition of plant and animal communities and may cause changes in soils, hydrology, fire regime, nutrient cycling, or other habitat characteristics, thus affecting the abundance of many other species (e.g. Cox, 1999). Pollution and alteration of habitats commonly favor invasive over noninvasive plants. Invasive plants are an important concern in the Meadowlands, where common reed and mugwort dominate thousands of hectares, and tree-of-heaven (Ailanthus altissima), princess tree (Paulownia tomentosa), white mulberry (Morus alba), black locust (Robinia pseudoacacia), Himalayan blackberry (Rubus discolor), Japanese knotweed, purple loosestrife, and other exotics are abundant. Although hard data are lacking, some native plants are probably absent from the Meadowlands due to the proliferation of invasives.

Figure 6. Purple loosestrife (Lythrum salicaria) stand where death of common reed (Phragmites australis) has caused a peat mass to float to the surface in Kearny Marsh West, Hackensack Meadowlands, New Jersey. This loosestrife was attended by several species of butterflies as well as other flower visitors. Photograph by Erik Kiviat.
Common reed is believed to build up tidal marsh surfaces and fill in headwater tidal creeks that fish, crabs, and grass shrimp use to move between marshes and open estuary (Weinstein & Balletto, 1999; Windham & Lathrop, 1999; Able & Hagan, 2000; Rooth & Stevenson, 2000). Large-scale wetland-restoration projects in the Meadowlands have aimed to remove or reduce reed stands and increase tidal flushing to improve access to the marshes for estuarine fishes and nektonic invertebrates, as well as to increase mudflat or pond habitat (Kiviat & MacDonald, 2002). Nonetheless, there are few data demonstrating the reed's effects on soils, hydrology, and plant and animal life specifically in the Meadowlands where wetlands are more degraded than in reed study areas elsewhere (see Kiviat & MacDonald, 2002). Common reed has been shown to have both negative and positive effects on habitats and biodiversity in the Meadowlands and elsewhere in the northeastern states (Kiviat & MacDonald, 2002). Monitoring data show that reed removal has reduced the number of birds that breed in reed marshes and that those birds have been partly replaced by the foraging and migrant birds of the new (intertidal cordgrass marsh, pool, or mudflat) habitats (A. Seigel, personal communication, 2004).
The Built Environment
Some native species benefit from the built environment. The chimney swift (Chaetura pelagica) is associated with increased building densities in other regions (Savard & Falls, 2001). The peregrine falcon (Falco peregrinus) benefits from increased abundance of prey in urban habitats, such as rock pigeons (Columba livia) and European starlings (Sturnus vulgaris) (Jenkins & Avery, 1999). In or near the Meadowlands, native species that have been observed using the built environment include woodchuck (Marmota monax), denning in berms and landfill cover; least tern, nesting on a roof; peregrine falcon, nesting on bridges and buildings; barn swallow (Hirundo rustica), nesting in observation blinds and under bridges; diamondback terrapin, nesting on highway shoulders), and native bees, nesting and foraging at inactive landfills. Trash and dumps support many native species including American woodcock (Scolopax minor), brown snake (Storeria dekayi), milk snake (Lampropeltis triangulum), and the land snail Zonitoides nitidus.
The built environment also has negative influences on wildlife populations and their distribution in the Meadowlands. It has been estimated that buildings and windows account for as many as 980 million bird deaths, power lines up to 174 million bird deaths, and communication towers as many as 50 million deaths annually in the U.S. (Klem, 1990; Erickson et al., 2001). Tall, illuminated structures such as buildings and communication towers are a significant cause of death for nocturnally migrating songbirds (Taylor & Anderson, 1973; Taylor & Kershner, 1986; Crawford & Engstrom, 2001) and nonpasserine birds including green herons, rails, and coots (Taylor & Anderson, 1973; Seets & Bohlen, 1977). The impact of the many communications towers in the Meadowlands is being studied (N. Tsipoura, personal communication, 2004).
The effect of roads on mortality, reproduction, and species distribution in the Meadowlands has not been studied. Elsewhere, birds, mammals, reptiles, and amphibians suffer high levels of mortality from collisions with vehicles (Ashley & Robinson, 1996; Haxton, 2000; Mumme, Schoech & Woolfenden, 2000; Carr & Fahrig, 2001; Erickson et al., 2001; Gibbs & Shriver, 2002; Taylor, 2002). Forman, Reineking and Hersperger (2002) found that in areas of heavy traffic (more than 30,000 vehicles per day) the presence and breeding of grassland bird species was reduced as far as 1,200 meters from the roadway. That level of traffic is approached on several highways in the Meadowlands. Barrier fences have been erected at four locations to guide terrapins beneath the New Jersey Turnpike using existing waterways (Urffer, 2002).
Landscape Perspective
Urbanization causes habitat fragmentation, increases the diversity of habitat types in the landscape (Gilbert, 1989), and may also result in the preservation of large blocks of undevelopable habitat. The ability of species, populations, and communities to respond to natural and uniquely urban conditions is largely moderated by the spatial configuration of habitats within the larger landscape, and by the scale at which individual organisms perceive the landscape.
The large expanses of wetlands in the Meadowlands, and the variety of types of wetlands and waterways, are the best explanations for the dense congregations and high diversity of marsh and water birds found there. The common reed marshes alone display great variety due to differences in tidal influence; depth and duration of standing water; stand size, density, and stature; stand shape and edge configuration; interspersion of reed with patches of other vegetation and pools of open water; mixture of other plants within and at the edges of the reed patches; lodging or falling over of reeds in storms; muskrat activities; fire effects; all-terrain-vehicle trails; and the surrounding landscape (Kiviat & MacDonald, 2002; Kiviat, personal observation). Extensive stands of reed or other nonforest vegetation are necessary to support nesting of the northern harrier, a bird that requires large areas of contiguous habitat and is sensitive to human intrusion near its nest.
Although larger wetlands are better habitat for many organisms, small isolated wetlands or ponds are better for amphibian larvae, clam shrimp, and other animals that are sensitive to competition or predation, and these wetlands also act as "stepping stones," connecting other wetlands (Semlitsch & Bodie, 1998). This "gain" in habitat diversity is, of course, offset by the historic loss of large areas of salt meadow, Atlantic white cedar swamp, and natural upland habitat in the Meadowlands.
The need for habitat connectivity is species-specific and varies with the scale at which organisms are able to use a landscape (Hostetler, 2001). Wide-ranging animals such as red fox (Vulpes vulpes) and northern harrier in the Meadowlands require large areas in order to locate adequate prey. However, even species with small ranges typically require large or connected habitats for dispersal, immigration, emigration, exploitation of patchy resources, and, in the case of a tidal system such as the Meadowlands, daily migrations necessitated by rising and falling water levels. Shorebirds, waterfowl, and wading birds are highly mobile and use a spatially dispersed complex of habitats on tidal, daily, and seasonal cycles (Kiviat, 1989; Haig, Mehlman & Oring, 1998). Particular species may focus their activities in small areas of the Meadowlands or range across the entire New York–New Jersey Harbor estuary.
Maintenance of locally and regionally diverse wetland complexes may be an important factor in conserving waterbird diversity. Many Meadowlands animals require adjacent upland and wetland habitats. Some species use wetlands and waterways for foraging but nest in adjacent, dry uplands (for example, the diamondback terrapin). Many shorebird species move into adjacent shallow water and mudflat areas when water levels in impoundments or tidal marshes become too deep (Kane & Githens, 1997). More highly mobile species such as bats, canids, waterfowl, herons, and dragonflies should be able to exploit the fragmented Meadowlands landscape more effectively than small terrestrial mammals, reptiles, amphibians, and snails, whose ability to move among habitat patches is limited and whose risk of mortality during migration or dispersal is greater.
Management Implications
1. Species diversity in the Meadowlands requires maintaining a diversity of habitats. The preservation of all the major wetlands in the Meadowlands district has been proposed, and there is interest in preservation along the upper Hackensack River estuary between the northern end of the district and Oradell Dam (T. Schvejda, personal communication, 2004). In addition to the better-known tidal, impounded, and nontidal wetlands, conservation planning should consider other habitats that support biodiversity in the Meadowlands. This biodiversity is not limited to large wetlands, tidal wetlands, cordgrass marshes, or restored sites: Thirteen of the 33 listed bird species are associated with upland habitats, and wetland wildlife needs upland buffer zones. Therefore, preserving the wetlands alone will not protect all the resources needed by the listed birds (and many other species). Very little is known about habitat combinations required by native animals for the avoidance of predators and during different life stages, seasons, and weather and food-availability conditions; upland-wetland complementary resource use is crucial for many species and needs to be considered during planning.
Habitats such as the oligohaline tidal marshes and dry sand scrub of the upper Hackensack River estuary and the puddles supporting clam shrimp should also be considered for conservation purposes, as should rocky upland habitats, which are important for terrestrial biodiversity. Which landfills should be maintained as green space and how they should be managed (e.g., for native pollinators and birds of prey) should be assessed; one possible model is the grassland bird habitat that has been successfully developed on the large, capped Croton Point Landfill in Westchester County, New York. It is also important to know which habitats in the Meadowlands support a diverse community of native pollinators and to protect these habitats or ensure that developed areas such as golf courses provide a functional replacement. New golf courses should be designed for low environmental impact (with integrated pest management, xeriscaping, low fertilizer input, and out-of-play areas designed to provide habitat for native pollinators, the American woodcock, and other native species of conservation concern).
In the Meadowlands, there are three areas that are as much as a kilometer from the nearest railroad or public road. This isolation from human disturbance is remarkable in the New York metropolitan area. In addition, examination of aerial photographs shows continuous major wetlands along the entire north-south length of the Meadowlands west of the Hackensack River. Proximity to roads and fragmentation of Meadowlands habitats by roads and railways undoubtedly restricts the presence or reproduction of many animals, some of them rarities (although it may also restrict the number of predators, white-tailed deer, or humans in marsh interiors, thus releasing certain other species from the pressures of predation, competition, or human intrusion). Some herons and the northern harrier, for example, are sensitive to human activities and predators near their nesting sites. For these reasons, larger and more isolated sites are important in conserving biodiversity. However, small habitat fragments provide locally important habitat for certain plants or invertebrates. For example, the native hackberry tree (Celtis occidentalis), which occurs in small local populations, potentially supports two uncommon Asterocampa butterfly species.
2. The costs and benefits of common reed need to be weighed objectively. One of the most contentious and complex issues in the Meadowlands is the management of common reed stands (see Kiviat & MacDonald, 2002). Reed stands in the Meadowlands are highly varied, and their associated organisms are not well understood, although there is evidence that they support rare species as well as common species of amenity value (i.e., nonmaterial value, such as aesthetic). Reed management methods used in other regions, for example, in the British Isles (Hawke & José, 1996) and the Delta Marsh in Manitoba, Canada (Ward, 1942), and may prove useful in altering the characteristics of Meadowlands reed stands to accomplish specific goals of conserving biodiversity and ecosystem services. Based on what is known about how reed stands affect Meadowlands biodiversity, we believe the recent emphasis on controlling the reed with herbicide, lowering and recontouring substrates to enhance tidal flushing, creating ponds and islands, and planting smooth cordgrass (Scarlatelli, 1997; HMDC, 1999; Doss, 2000; Hartman, 2003) is too narrow an approach. For example, such controls favor estuarine benthos, estuarine fishes, and migrant waterbirds and shorebirds over terrestrial invertebrates, breeding birds, and mammals; they don't take into consideration the species of vascular plants and invertebrates associated with reed stands (Kane, 2001a, b); they ignore the potential impact of remobilizing sediment and toxic contaminants; and they don't account for the future maintenance costs associated with sea-level rise or reinvasion of common reed. The habitat functions and ecological services associated with existing stands of invasive plants must be assessed objectively and weighed against the potential costs of restoration, nontarget impacts, long-term management, and unknowns associated with attempts to trade, for example, common reed for cordgrass, cattail (Typha species), submergent aquatic plants, or mudflats. When the reed stands are used by numerous endangered, rare, declining, or vulnerable species, as is the case in the Meadowlands, the assessment and decision-making process should be nuanced, and science and values should not be confused. Whatever the mixture of introduced and native plants, it may be less expensive and environmentally risky to maintain an existing community that is providing habitat and amenity in an urban environment than to attempt to create or re-create a community of native species that once may have existed in the area or that now exists in a more rural or wild environment elsewhere (Gilbert, 1999).
3. Specific prescriptions for management. The biodiversity value of the Meadowlands raises questions about how the area might be managed to maintain and improve habitat for species of concern. We discuss here a few important opportunities for improving habitat to enhance biodiversity.
There are old drainage ditches still drying out the swamp forest and wet meadows at Teterboro Airport (Berger Group, 2000), apparently compromising their ability to support biodiversity (MacDonald, personal observation). The ditches could be plugged and hydrology restored. Mowing (frequent for a few years, then less often, and avoiding the bird-nesting season) could improve grassland bird habitat in selected portions of the large areas of inactive landfill dominated by mugwort and upland common reed stands. Forest cover could be established (Robinson & Handel, 2000) or shrubs and quaking aspen planted for woodcock. Planting nectar plants in parks, medians, yards, and recreation areas could enhance the landscape for native bees, butterflies, and other flower-visiting insects. The water level in Kearny Marsh West apparently needs to be lowered to prevent further deterioration of some common reed stands, with the goal of achieving extensive areas of reed with numerous small and large shallow pools for water and marsh bird breeding and foraging habitat. Existing small stands of purple loosestrife that have developed on floating mats of peat and dead reed rhizomes support a diverse community including mosses and nectar-seeking butterflies. The loosestrife stands increase the biological diversity of the marsh and should be left unless they become very extensive or very dense and the associated biota becomes species-poor. Large wet meadows dominated by common reed, such as portions of the Empire Tract, would benefit from excavation of shallow pools about 50 to 100 meters in diameter for marsh and water birds as well as other wildlife. Mowing or prescribed livestock grazing of reed between the bluejoint (Calamagrostis canadensis) meadows on the west side of the Paterson Lateral gas pipeline might be used to maintain and expand these meadows. To avoid further damage to adjoining marshes, off-road vehicles need to be confined to the pipeline road, where they could be used to maintain the existing clam shrimp pools on the road surface.
Siting, height limits, and lighting of tall structures in the Meadowlands should be based upon the best available information for minimizing bird collisions. Unlit, un-guyed towers less than 60 meters tall pose the least threat to migrating birds (Manville & Evans, 2000). The Federal Aviation Administration requires aviation safety lighting on structures 60 meters or taller. On these structures, solid red or blinking incandescent aviation lighting appears to be more of a hazard than white flashing strobes (Manville & Evans, 2000). Siting of these tall structures has a major influence on bird mortality. According to Manville (2000), the worst-case scenario includes having tall structures next to a wetland, a major songbird migration corridor, and fog. The Meadowlands meet at least the first and second criteria, and probably the third. In addition, power lines adjacent to wetlands are a known hazard to waterbirds taking off and landing in these habitats, and they should also be considered a problem in the Meadowlands.
Certain species are missing from the Meadowlands. In some cases these are species with limited ability to disperse from source populations outside the Meadowlands. There may be species for which reintroduction would make sense; a large-scale turtle reintroduction experiment at Floyd Bennett Field in Brooklyn, New York, part of the Gateway National Recreation Area, may provide a model (Cook, 1996). It may also be helpful to create corridors or highway crossings designed for particular organisms.

Figure 8. Atlantic white cedar (Chamaecyparis thyoides) stump at the Mill Creek mitigation site, Hackensack Meadowlands, New Jersey. All that persists of once-extensive cedar swamps, such stumps could support interesting invertebrates that may be specialized to this microhabitat. Photograph by Erik Kiviat.
4. The varied human uses of Meadowlands plants and animals should be considered. Wildlife is the focus of much recreational and educational activity, notably observation of birds and to a lesser extent mammals, butterflies, dragonflies, and wildflowers. For example, sportfishing is common on the Hackensack River main stem and a few accessible tributaries. People also find practical uses for wild organisms, and we increasingly discover or develop ways to use them as foods, pharmaceuticals, specialty woods, fuels, industrial feedstocks, and pesticides. Among Meadowlands species that may prove useful in the future are the oyster mushroom (Pleurotus sapidus), for food; the princess tree, as a high-quality hardwood; common reed, for thatch, energy, and fiber; and Japanese knotweed, for medicine. Biodiversity managers must be aware of the potential for overexploitation of native species such as large fungi; management of invasive species through consumption; public exposure to contaminants or pathogens through the use of wild plants and animals; and ecological side effects of harvesting these organisms.
5. Research is needed to study the effects of contaminants and urban habitats on species survival and productivity. Although the Meadowlands exhibit a high diversity of wildlife, decision makers would benefit from a more detailed assessment of how artificial and altered habitats, as well as fragments of seminatural habitats, meet the environmental tolerances and ecological needs of organisms. In particular, studies of whether such habitats may be hazardous or unhealthy for certain species (for example, the northern water snake, night herons, raptors, mink) are needed. Such information would allow for more effective conservation and ecological restoration.
6. Additional taxonomic survey work is needed. Better data would enable restoration, management, and development planning to proceed with sensitivity to the Meadowlands' special biological values. Missing information and lack of survey coverage for many taxa and habitats inhibit the understanding of Meadowlands biodiversity. This is especially true for smaller animals and plants, and for organisms of habitats other than marshes. Among these less-studied habitats are the meadows, scrub, and swamp forests of the northern Meadowlands, including wet clay meadows and bluejoint meadows; small or temporary pools; storm-water ponds; artificial habitats such as mines, landfills, and other wetland fill; habitats associated with invasive plants such as tree-of-heaven, princess tree, mugwort, Japanese knotweed, and purple loosestrife, as well as common reed; and habitat fragments enclosed by built environments such as highway intersections, railroads, parking lots, and buildings. The relict native plants and plant communities in many areas need to be studied. We also need to understand larger-scale space use by mobile animals in relation to habitats and hazards (e.g., highways, antennas, contaminant hot spots). Where it is determined that mortality or morbidity associated with anthropogenic hazards is unacceptable for particular species, the built environment or other habitats can be modified to reduce the exposure of those species to risk.
Applicability to Other Urban Wetland Complexes
The Meadowlands contain a large area of degraded wetlands that support numerous species, many of which are rare or vulnerable. In this respect, the area is similar to other urban wetland complexes, such as Jamaica Bay Wildlife Refuge in New York City, a component of Gateway National Recreation Area (Tanacredi, 1995), Union Bay in Seattle (Higman & Larrison, 1951), Tinicum Marsh in Philadelphia (McCormick, Grant & Patrick, 1970), and the San Francisco Bay estuary complex (Josselyn, 1983). Because rich natural resources and natural transportation corridors have supported the development of many "wetland cities" around the world (Kiviat, 1991), we expect there are many biodiverse urban wetland complexes elsewhere. In general, although each locality has its own species and unique problems, they are all subject to fragmentation, contamination, poor water quality, and other hazards. Urban wetland complexes are inhospitable to many nonflying, area-sensitive animals (i.e., animals that need large areas of contiguous habitat); organisms sensitive to pollutants such as PCBs, heavy metals, or airborne sulfur; and organisms requiring habitats that are scarce in urbanized areas, such as natural upland soils, unaltered streams, or forest interiors. However, they should be favorable for migratory animals that move along riverine or coastal corridors; marsh and water birds requiring isolation from human intrusion; raptors preying on peridomestic small mammals or birds; and organisms that tolerate urban conditions and benefit from reduced grazing, predation, or competition.
The creation and maintenance of large blocks of aquatic and terrestrial habitat interconnected by suitable corridors for many of the organisms that otherwise tolerate urban conditions are high priorities for landscape planning and management. Yet smaller habitat fragments are also important. Urban wetlands should be viewed as habitat both for rare species of general conservation significance and for common species that nonetheless may be an important amenity for urban humans.
*Except where noted, measurements throughout this paper are in metric notation; conversions to U.S. equivalents can be obtained at http://www.onlineconversion.com/length.htm.
Acknowledgments
We are grateful to many individuals in nongovernmental organizations, educational institutions, businesses, and public agencies who have made information available to us and helped in other ways (see acknowledgments in Kiviat & MacDonald [2002]). Our work has been supported by the Mary Jean and Frank P. Smeal Foundation, H2O Fund (Highlands to Ocean Fund), Geraldine R. Dodge Foundation, Geoffrey C. Hughes Foundation, Natural Resources Defense Council, and Hackensack Meadowlands Partnership. This is Bard College Field Station-Hudsonia Contribution 85.




