Growth and Reproduction of Eastern Oysters, Crassostrea Virginica, in a New York City Estuary: Implications for Restoration
by Chester B. Zarnoch1,2 and Martin P. Schreibman2
1 Department of Natural Science, Baruch College CUNY, 17 Lexington Avenue, New York, NY 10010
2 Aquatic Research and Environmental Assessment Center, Brooklyn College CUNY, 2900 Bedford Avenue, Brooklyn, NY 11210
Abstract
The Hudson River estuary (HRE) had significant populations of eastern oysters (Crassostrea virginica) prior to the 1920s. A combination of overfishing, pollution, and habitat destruction led to the loss of both the oyster fishery and the ecological services oysters provide. Recent improvements in water quality have led to an interest in reintroducing eastern oysters into the HRE to enhance ecosystem characteristics. However, there are few data on oyster biology and the potential for their restoration in the HRE. In this study, we measured growth, reproduction, and survival of oysters transplanted to two sites in Jamaica Bay (New York) with contrasting water-quality parameters. Condition index and gonadal stage were measured in adult oysters from May through August 2003. Juvenile growth and mortality were measured from July to October 2003. These measurements provide the first description of oyster growth, survival, and reproduction in the HRE. We found that adult oysters successfully spawn in Jamaica Bay, and their gonadal maturation is similar to that of oysters in Long Island Sound. Juvenile growth, measured as shell height (mm), was comparable to that of oysters in other local marine systems and was not affected by the reduced water quality in Jamaica Bay. These preliminary data can guide restoration planning. However, we suggest that several issues need further research before restoration efforts are implemented in the HRE.
Keywords: Hudson River estuary, eastern oyster, Jamaica Bay, restoration, growth, reproduction, survival
Introduction:
Oysters in New York City
Eastern oysters (Crassostrea virginica) served as a major source of food and industry for the New York area for more than two hundred years. Prior to 1900, oysters were found from the lower Hudson River estuary (HRE) to as far north as Haverstraw Bay, NY. Jamaica Bay, an important estuary in New York City, provided such optimal growing conditions that small oysters (seed) could reach market size in one season. Jamaica Bay was said to have produced up to 700,000 bushels of oysters per year during its peak (Franz 1982), which was likely between 1900 and 1910. In 1904, the harvest was worth approximately $603,000. In 1906, the Jamaica Bay clam and oyster fisheries had a value of $2,000,000 (Black 1981). However, by the 1920s the HRE oyster industry was decimated due to overfishing, habitat loss from dredging and filling, and pollution. Sewage was likely the most significant factor contributing to oyster population loss, as eutrophic conditions stimulated low dissolved oxygen levels (Franz 1982). Oyster fisheries in NYC waters were shut down in 1921 due to public health concerns about the consumption of sewage-contaminated oysters (U.S. Works Progress Administration 1939). All shellfisheries in New York City remain closed to this day due to poor water quality. Currently, there are very few oysters living in the HRE (Steimle 2005). A report from the National Oceanic and Atmospheric Administration (NOAA)'s Eastern Oyster Biological Review Team (2007) described oysters in the HRE as "ecologically extinct," since they no longer act as a keystone species or provide ecosystem services.
Water quality has recently improved in the HRE as a result of legislation and increased sewage treatment (Brosnan et al. 2006). Enhanced water quality has stimulated interest in reviving oyster populations. However, there are potential problems with oyster restoration efforts from a resource management perspective. Increasing oyster abundance in the HRE may lead to increased poaching (it is illegal to harvest shellfish from NYC waters), and oysters could become a public health risk. In addition, dredging and filling activities have removed most historical oyster beds, resulting in a lack of existing substrate for larval settlement. Therefore, oyster restoration efforts will have to include construction of new reefs, and these likely will replace existing soft-sediment benthic communities (Barnes 2005).
Water quality in New York City
New York City's Department of Environmental Protection (NYCDEP) recently published a management plan to improve water quality in Jamaica Bay (Jamaica Bay Watershed Protection Plan 2007). A significant cause of the impaired water quality is the addition of 15,800 kg nitrogen (N) per day into the bay, 89% of which originates from four wastewater treatment plants that surround the bay (Benotti et al. 2007). Similar nutrient loading occurs throughout the HRE, particularly in the East River and western Long Island Sound (O'Shea and Brosnan 2000). Nitrogen loading enhances primary production and algae blooms. Subsequent decomposition of algae results in low-oxygen "dead zones," which negatively affect fisheries (Kennish 2002; Halpern et al. 2008). Enhancing the ability of aquatic organisms to recycle and remove excess nutrients is of major interest for both ecological and public health (Alexander et al. 2000; Johnson et al. 2008).
Oyster restoration as a management strategy
Oysters deliver nutrients and carbon resources from the water column to the benthos via the release of biodeposits in the form of feces and pseudofeces (filtered particles that are rejected prior to ingestion). This process decreases the turbidity of the water and stimulates the growth of microphytobenthos such as microscopic, photosynthetic eukaryotic algae (diatoms) and cyanobacteria, and of submerged aquatic vegetation, which stabilizes sediments and increases habitat available for benthic fauna. Nutrients and organic matter from bivalve wastes are then mineralized by sediment microbes (Newell 2004). Mineralized nitrogen can be reabsorbed by the phytoplankton, taken up by aquatic vegetation and microphytobenthic flora, or removed through denitrification. Thus, enhancement of oyster stocks has been proposed as a restoration strategy to improve water quality (Brumbaugh et al. 2000; Nelson et al. 2004; Coen et al. 2007). Restoration of oyster reefs may also improve secondary production, such as finfish and crustaceans, by providing a complex habitat (Harding and Mann 1999; Peterson et al. 2003). Furthermore, small fringing reefs can be used to protect shorelines in low-energy environments (Piazza et al. 2005). Therefore, the restoration of oyster reefs has been identified as an important goal for enhancing the entire HRE ecosystem (Bain et al. 2007). The Army Corps of Engineers, NY/NJ Port Authority, and NYCDEP have suggested oyster restoration as a strategy for improving ecosystem function. Oyster reef pilot studies are a component of the Jamaica Bay Watershed Protection Plan (2007). In addition, the NY State Department of Environmental Conservation (NYSDEC) (2007) stated that reestablishing oyster populations in the estuary is a priority.
Although a recent comprehensive study of Jamaica Bay has shown it to be a much improved and quite productive estuary (Tanacredi et al. 2002), it is still challenged by nutrient loading and lacks prominent oyster populations. To obtain the ecological benefits of restored oyster reefs, the basic requirements of appropriate water quality and substrate must be met (Breitburg et al. 2000). The lack of significant oyster populations in the HRE even with no commercial or recreational harvesting suggests possible limitations, which may include a lack of larval substrate, depensation (reduced reproductive success at low population densities), poor water quality, and disease. However, there have been no published studies of oyster populations and their potential restoration in the HRE. To address this dearth of information, we measured survival, growth, and reproduction of oysters transplanted to two sites in Jamaica Bay with contrasting water-quality parameters. The study was intended to collect preliminary data that can guide future restoration efforts in the HRE.
Methods:
Study sites
Both study sites were located in Jamaica Bay (a component of the HRE), an approximately 4,000-hectare urban estuary located at the southwestern end of Long Island, New York (Franz and Tanacredi 1993). The two field sites were at Floyd Bennett Field (FBF; N40°36.380"W073°53.128") and near the mouth of Bergen Basin (BB; N40°38.732"W07349.216"). All samples (oysters and water) were collected within 1 hour of mean low water (MLW) at each site. The depth of the FBF site at MLW is approximately 1.0 meter. The depth of the BB site at MLW is approximately 0.5 meter. These sites were chosen to represent both adequate (FBF) and poor (BB) water quality conditions. FBF is located at the southwestern end of the bay, close to the bay's entrance. BB is located in the northern part of the bay. Nutrient levels (i.e., nitrate and phosphate) are consistently higher in the northern parts of the bay than in the well-mixed southern channel closest to the bay entrance (Sambrotto 2002; Ringenary 2008). Spatially and temporally mediated hypoxic events have occurred sporadically in the northern portions of the bay. The mouth of Bergen Basin in particular appears to have reduced dissolved oxygen levels during summer months (Ringenary 2005; Ringenary 2008).
Adult reproduction
Approximately 200 adult oysters were obtained from a New York shellfish grower in April 2003 and held in a floating cage in Rockaway Inlet until they were deployed in Jamaica Bay. The oysters were ~80 mm in shell height and estimated to be 2 to 3 years old. Shell height is measured as the distance from the end of the umbo to the ventral shell margin (Galtsoff 1964). One hundred oysters were placed in three 1.9-centimeter mesh bags (107 cm × 61 cm × 8 cm), which were then deployed on 29 April 2003 at BB and 1 May 2003 at FBF. The bags were tied to a cinderblock and were kept directly on the benthos. Oysters (n = 10‒12) from each site were sampled twice per month through August 2003 to determine condition index and reproductive status. The mesh bags were replaced with new bags (identical) on each sample date to minimize the impact of fouling organisms, which reduce flow of water through the cage. Previous experiences of field work in Jamaica Bay suggested the possibility of vandalism of our deployed cages (Zarnoch and Schreibman 2008). In fact, within the first two weeks of deployment, the oyster bags, oysters, and cinderblock at BB went missing, thus substantiating the risks of working in an urban system. Results on adult reproduction are therefore reported for FBF only. Because of the health risks associated with consuming shellfish from Jamaica Bay, the small sample size used in our study was considered a tradeoff for reduced public health risks.
Oysters sampled from FBF were brought back to the laboratory, where they were measured with calipers to determine shell height. Whole oysters (n = 5‒6) were weighed with an electronic balance and carefully opened, and all tissue was removed. The wet tissue weight and wet shell weight were determined separately. The tissue and shell samples were then placed into a drying oven at 70°C for ≥ 48 hours, then reweighed to obtain the dry weight of each sample. The dried tissue from each sample was then ignited in a muffle furnace at 500°C for 24 hours, cooled in a desiccator, and reweighed to determine its ash-free dry weight. A condition index was calculated for each sample as the ratio of ash-free tissue dry weight to shell height × 100 (Lucas and Beninger 1985). The condition index is a measure of the metabolic condition of a bivalve and is related to the quantity of glycogen stored (Mann 1978; Lucas and Beninger 1985). An oyster's tissue content can increase or decrease in response to environmental or physiological parameters such as diseases, spawning, temperature, and food availability. Since the shell can only increase or remain the same in size, it is a useful reference material (Mann 1978). The greater the ratio of tissue weight to shell height, the greater the tissue content.
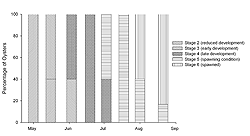
The remaining samples (n = 5‒7) were used to determine reproductive condition. A 4‒6-mm cross section of the gonad posterior to the junction of the gills and labial palps was removed from each oyster (Morales-Alamo and Mann 1989). Each portion was fixed in Bouin's solution, embedded in Polyfin™, sectioned transversely (7 μm), and stained with Masson trichrome stain (Presnell and Schreibman 1997). Histological sections were classified according to the different stages of gonadal development described by Kennedy and Krantz (1982) with the modifications of Quintana (2005). The stages include: 1) indifferent, 2) reduced development, 3) early development, 4) late development, 5) spawning condition, and 6) spawned. The percentage of samples occurring in each stage was calculated for all sampling dates.
Juvenile growth and survival
Approximately 2,000 juvenile oysters (about 8 mm in shell height) were obtained from the Frank M. Flowers and Sons shellfish hatchery (Bayville, NY). These oysters were separated into two groups of 1,000. Each group was deployed at the field sites in three 4.8-mm mesh bags (107 cm × 61 cm × 8 cm) at an approximate density of 333 bag-1 on 2 July 2003. The bags were attached to two cinderblocks and were kept directly on the benthos. The oysters were sampled twice each month until October 2003. When sampling each site, the oysters from all cages were combined, cleaned by sieving, and then redistributed evenly (as determined by volume) into three new cages. On the second sampling date, the 4.8-mm mesh cages were changed to 6.4-mm mesh cages. The shell height of a random subsample (n = 50) was determined using calipers. All measured oysters were returned to the cages. The instantaneous shell-height based growth coefficient of oysters (k) was calculated for July, August, and September using the equation k = (ln X2 ‒ ln X1) /(t2 ‒ t1) ×100, where k is the instantaneous growth rate, and X2 and X1 represent the shell heights in mm at times t2 and t1 (days) (Krebs 1972). The number of disarticulated valves and intact gaping valves were recorded and those oysters were removed from the cages. A daily mortality rate for each sampling period (Soletchnik et al. 2006) was calculated as [(initial number − final number/ final number + initial number)/2] /number of days ×100.
Water quality
Water parameters including temperature, salinity, and dissolved oxygen were determined for both sites on each sampling date using hand-held meters (YSI 85 and 550, respectively). Each meter was calibrated prior to sampling. Atmospheric conditions such as air temperature, wind speed, and direction were also recorded. Water samples were taken on each sampling date to measure chlorophyll-a and total ammonia nitrogen (NH3-N). These samples were collected near the benthos and kept on ice until analysis. Chlorophyll-a analysis was conducted on 500-ml samples using an acetone extraction method (Parsons et al. 1984). Chlorophyll-a is an indicator of phytoplankton biomass, since it is present in all photosynthetic eukaryotes, including the major groups of phytoplankton. Unionized ammonia (NH3-N) concentration was determined colorimetrically on 5-ml samples using Nessler's reagent (APHA 1992). NH3-N is dependent on pH, salinity, and temperature and is considered the most toxic form of ammonia.
Statistical analyses
Statistical analyses employed a one-way analysis of variance (ANOVA) to test for differences in shell length, dry tissue weight, ash-free dry weight, and condition index in the adult oysters. Tukey's Honestly Significant Difference (HSD) test was used when significant (p < 0.05) differences were found. A two-way repeated measures ANOVA was used to determine the influence of site, sampling time, and the interaction of site × sampling time on juvenile oyster growth. Normality and heterogeneity of variance were tested using the Kolmogorow‒Smirnov statistic and Levene's Test, respectively. Adequate transformations were performed when necessary. Statistical tests were conducted with SPSS version 16.0, and figures were created with SigmaPlot 8.0 (SPSS Inc., Chicago, IL).
Results:
Adult reproduction
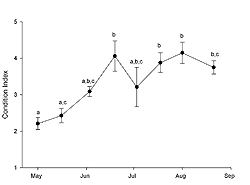
The oysters sampled at FBF significantly increased in condition index (Figure 1) from the initial May sample to the 19 June sample (ANOVA; p < 0.0001), which was also the greatest value observed. During this period, water temperature increased (Table 1) and the gonadal status (Figure 2) changed from stage 2 (reduced development) to stage 4 (late development). Condition index decreased at the first July sampling (Figure 1), which was also the first date we observed evidence of spawning in histological sections (Figure 2). Water temperature was approximately 22° C (Table 1) when spawning peaked in July (Figure 2). Evidence of active spawning was observed in samples through August. However, by the end of August, over 80% of the samples were categorized as spawned (Figure 2) and showed resorption of gametes. There was no statistically significant change in condition index (Tukey HSD; p < 0.05) from the 19 June sample through the rest of the sampling period (Figure 1). Cumulative mortality of the adult oysters held at FBF from May through August was 4% (data not shown).
Juvenile growth
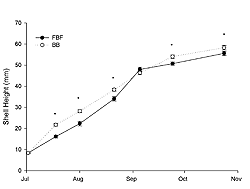
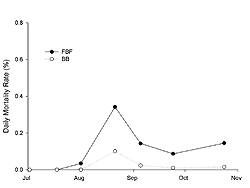
Juvenile oysters grew from an initial shell height of 8.37 mm (SE±0.15) in July to 55.58 mm (SE±0.99) and 58.28 mm (SE±1.02) in October at FBF and BB, respectively (Figure 3). Growth was significantly influenced by site, sampling time, and their interaction (two-way RM ANOVA; p < 0.0001). Shell height was significantly greater (ANOVA; p < 0.001) at BB on all sample dates except 9 September (ANOVA; p = 0.2). The instantaneous growth coefficients were greatest in July and then decreased through August and September (Table 2). The daily mortality rate was relatively low (< 0.4%) for both sites throughout the sampling period (Figure 4); however, observed mortality was higher at FBF. The greatest observed daily mortality rate occurred at the end of August at FBF. A subsequent analysis of dead oysters (n = 43) revealed that 81% had been killed by Urosalpinx cinera (oyster drills), as evidenced by drill holes. Oyster drills were also collected during sieving.
Water quality
All water quality data are presented in Table 1. During July and August, water temperature was greater at BB, while salinity was lower. Dissolved oxygen levels were variable during these months. A summer phytoplankton bloom occurred in August as indicated by an increase in chlorophyll-a. Chlorophyll-a values were higher at FBF on all sampling dates. Reduced dissolved oxygen levels were observed at BB in September as chlorophyll-a values decreased. The mean ammonia value was 1.74 mg l-1 at BB throughout the sampling period. This was approximately 3 times higher than values from FBF.
Discussion:
There is considerable interest from management agencies and nonprofit groups in restoring oysters to Jamaica Bay and the entire HRE (Bain et al. 2007; Jamaica Bay Watershed Protection Plan 2007; NYSDEC 2007) to gain the ecosystem services they provide (Brumbaugh and Toropova 2008). Despite this interest, there have been no prior studies on oysters in the HRE, and thus the potential for successful restoration efforts is largely unknown. Our study provides the first description of oyster growth, survival, and reproduction in the HRE.
The timing and duration of spawning in subtidal eastern oyster populations varies geographically. Oysters in Prince Edward Island (Canada) may spawn from late June through August (Kennedy and Battle 1964), while oysters from Wassaw Sound (Georgia, US) will spawn from April through September (Heffernan et al. 1989). Loosanoff (1942) reported that subtidal oysters in Long Island Sound (Connecticut, USA) will spawn in late June through August. When studying intertidal populations of eastern oysters in Long Island Sound, Brousseau (1995) found spawning to begin in late June or early July and continue through September. In our study, oysters held subtidally in Jamaica Bay spawned from July through August (Figure 2); thus, our results are consistent with those from Long Island Sound. Spawning initiated in July when water temperatures were approximately 22° C. Subtidal oysters in Long Island Sound may spawn at temperatures under 15° C (Loosanoff and Engle 1940), while intertidal populations begin spawning at 20° C (Brousseau 1995). Although there may be factors other than temperature that trigger spawning, it appears as though oysters held subtidally in Jamaica Bay require temperatures similar to the intertidal populations of Long Island Sound to induce spawning.
Juvenile oysters grew well at both sites (Figure 4), with relative growth rates highest in July (Table 2). Growth observed in Jamaica Bay is comparable to that at other local systems during a similar time frame (Table 3). In addition, instantaneous growth coefficients for shell height in Jamaica Bay were similar to coefficients determined from oysters grown in Oyster Bay (Bricelj et al. 1992), which is on the north shore of Long Island (NY). Oysters held at BB were generally larger than those held at FBF (Figure 4). There are many factors that could influence the growth of oysters. Temperature and food supply are often considered the most important (Newell and Langdon 1996; Shumway 1996). Food supply, as estimated by chlorophyll-a, was greater at FBF throughout the sampling period but was not limited at BB, as it ranged from 3.2 to 10.25 μg l-1. Growth rate of eastern oysters is affected by temperature and latitude, with growth greatest in low latitudes due to the warmer water (Shumway 1996). In July and August of our study, water temperature was higher at BB (Table 1), and it is likely that this influenced growth at BB. Poor water quality at BB, as evidenced by high NH3-N and reduced dissolved oxygen (Table 1), did not impair growth. Oysters have the ability to regulate oxygen consumption under conditions of reduced oxygen tension, particularly at the salinities observed in our study (Shumway and Koehn 1982). Therefore it is not surprising that the reduced dissolved oxygen levels observed at BB did not impair growth. Measured NH3-N was as high as 2.0 mg l-1 at BB. Oysters, however, are highly tolerant of NH3-N, as compared to other marine species. Epifanio and Srna (1975) evaluated the toxicity of ammonia to oysters and found that a concentration of 6.0 mg l-1 for 96 hours will result in 50% mortality. However, the more toxic form (NH3) may become prevalent under basic conditions of pH, such as during phytoplankton blooms and associated photosynthetic activity (Soletchnik et al. 2005). Future studies should consider this issue. Jamaica Bay may be particularly sensitive to this phenomenon as it experiences significant phytoplankton blooms with chlorophyll-a values often exceeding 100 μg l-1 (Sambrotto 2002; Zarnoch and Schreibman 2008), and at times these blooms can become CO2-limited, as compared to other systems that are generally limited by nitrogen or phosphorous (Sambrotto 2002). The synergistic effects of high ammonia, reduced dissolved oxygen, and high contaminant levels found at BB or other sites within the HRE might reduce growth when oysters are exposed to these conditions over long time periods.
Juvenile mortality was low throughout the sampling period (Figure 4). Mortalities of juvenile oysters can be as high as 100% in populations affected by disease (Bricelj et al. 1992; Davis and Barber 1999; Ford and Borrero 2001). Although disease monitoring was not conducted in our study, it is unlikely disease agents were responsible for mortalities observed in Jamaica Bay as there were no obvious shell abnormalities (Howard et al. 2004) or reduction in shell growth. In addition, we observed oyster drill holes in 81% of the dead oysters. The mesh openings (6.4 mm) of the culture bags were large enough to allow the drills to enter and feed upon the oysters. Oyster drill feeding activity is greatest at a temperature of 25° C and salinity of 26.5 parts per thousand (Manzi 1970), both of which are similar to values observed in Jamaica Bay when the greatest mortality was observed (Table 1; Figure 4). Oyster drill predation on juvenile oysters can be quite significant, ranging from 0 to 5 oysters drilled day-1 (Cole 1942; Carriker 1955). Mortality was higher at FBF than at BB across all sampling dates. This may have been due to the proximity (< 5 meters) of the oyster cages to a wrecked boat which was encrusted with barnacles (Balanus spp; Semibalanus spp). Oyster drills were observed feeding on these barnacles and may have been attracted to the oysters via chemical cues. FBF may also have higher densities of U. cinera due to its hard substrate, which is preferred by this species (WAPORA 1982).
Conclusion:
Our study demonstrates that juvenile oysters transplanted into Jamaica Bay for restoration purposes are likely to survive and grow. In addition, adult oysters will follow a gonadal cycle typical of the NY area, so reproductive potential will not be limited by water quality. Although this bodes well for future restoration efforts aimed at improving water quality and ecosystem function, there are a number of significant issues from both research and management perspectives that need to be addressed in order for these efforts to be successful. For example, there is a lack of adequate substrate for larval settlement throughout most of the HRE due to dredging and filling activities (Franz 1982). Therefore, restoration efforts will have to include large-scale shelling programs to provide adequate substrate in the form of reefs. Decisions on where to site oyster reefs will have to consider evidence for historic oyster populations and potential larval advection/retention, of which little is known in the HRE. Shell-based reefs will need to be stocked with oysters at a density that will maximize fertilization. Mortality is likely to occur due to predation and disease (i.e., MSX and dermo); thus, reefs should be continuously monitored and stocked to maintain optimal density. In order to achieve improvements in water quality, oyster restoration should be conducted in areas where reefs can positively influence sediment nitrogen cycling (i.e., coupled nitrification-denitrification). The environmental parameters and oyster densities that will promote sediment denitrification processes have not yet been determined. To improve secondary production through oyster restoration efforts, the reef characteristics that encourage use by higher trophic levels will need to be determined. In addition, we speculate that the loss of our adult oysters deployed at BB was the result of a recreational fisherman. Future restoration efforts will have to include surveillance/monitoring programs and public outreach to educate recreational users about the health risks associated with consuming shellfish from the HRE. Finally, the massive nutrient loading from wastewater treatment plants will not be mitigated by restored ecological processes alone. Significant improvements in filtration and volume capacity at wastewater treatment plants must be coupled with ecological restoration efforts.
It is clearly evident that future restoration efforts in the HRE will have to be preceded by rigorous research programs to address these issues. Initiatives or management actions that fail to consider them are not likely to achieve measurable improvements in water quality and secondary production. Oyster restoration in Chesapeake Bay has been conducted for many years, arguably without significant improvements in ecological services (Mann and Powell 2007). Mann and Powell state that this is a result of poor management decisions and a lack of consideration of the population dynamics and ecology of oysters in this system. Oyster populations have been studied in Chesapeake Bay for over a century (Rothschild et al. 1994). In contrast, this manuscript is the first description of oyster biology in the HRE. There is clearly a need for more research to precede management efforts towards oyster restoration in the HRE.
Acknowledgments:
We would like to thank Gateway National Recreation Area for permission to work within the park and for the use of their research vessel. We are also indebted to the staff of the Jamaica Bay Wildlife Refuge and the Jamaica Bay Guardian for their assistance in field collections. The New York/New Jersey Port Authority Police aided in our sampling near Bergen Basin. We thank the Frank M. Flowers and Sons shellfish hatchery for providing juvenile oysters. The field and laboratory assistance of Stephano Diomede, James McMahon, Joseph Burnett, Doug Laing, Robert Dickie and Sandy Gamss is greatly appreciated. This study was supported by the City University of New York.