The Floristic Composition and Community Structure of the Forest Park Woodland, Queens County, New York
by Carsten W. Glaeser
City University of New York, Herbert H. Lehman College, Department of Biological Sciences, 250 Bedford Park Blvd., Bronx, NY 10468
Abstract
In 2000, a census was conducted within a 167-hectare wooded section of Forest Park, in Queens County, New York, to document the current floristic composition and structure of the woodland community. All woody stems ≥ 2.0 centimeters (cm) diameter at breast height (DBH) within a permanent and contiguous 0.5-hectare (50 × 100 meters) plot were identified, recorded, and measured for diameter, height, and x, y coordinates. The plot contained 771 stems from 22 woody species (15 genera and 13 families) reflecting a Shannon-Wiener index of 2.17 and a Simpson's index of 0.162. Five species were singletons, and three species were nonnative invasives. Stem DBH ranged from 2.0 to 116.7 cm, with a mean of 8.55 cm, and stem density was 1,542 stems per hectare. The largest-diameter trees were the oaks: red oak (Quercus rubra L.), black oak (Q. velutina Lam.), and white oak (Q. alba L.) (Fagaceae). The census revealed a young tree population largely dominated by characteristic pioneer species such as sweet birch (Betula lenta L., Betulaceae), black cherry (Prunus serotina Ehrh., Rosaceae), and the nonnative invasive Amur corktree (Phellodendron amurense Rupr., Rutaceae). The top dominant taxa based on Forest Inventory and Analysis importance values (IV) were Betula lenta, Quercus rubra, Phellodendron amurense, Cornus florida L. (flowering dogwood, Cornaceae), and Prunus serotina, and the dominant arborescent family was Fagaceae, represented by Quercus rubra, Q. velutina, Castanea dentata (Marshall) Borkh. (American chestnut), and Fagus grandifolia Ehrh. (American beech). The top dominant taxa based on importance values within the small-diameter class were Betula lenta, Phellodendron amurense, Cornus florida, and Prunus serotina. The top dominant taxa within the large-diameter size class were Quercus rubra, Betula lenta, Q. velutina, and Cornus florida. Ecological dominance in this urban woodland is shifting away from its historical legacy as an oak-hickory forest. The observed disturbance patterns, the decline in traditional dominant tree species, the abundance of pioneer tree species across the diameter-size classes, and the continued colonization by Phellodendron amurense may be weighted factors imposing structural change throughout the woodland.
Key words: ecological dominance hierarchy; fragmented forests; forest census; frequency distribution; nonnative invasive species; pioneer species; randomization tests; species importance values; stem-size class; urban forest ecology
Introduction
From the late 19th century through the 20th century, development along the urban-suburban interface altered much of the original landscape on western Long Island, New York. The New York metropolitan region, including outer boroughs such as Queens County, is now devoid of much natural landscape; nevertheless, it may contain more than 3,000 species of vascular plants (Brooklyn Botanic Garden, 1999). These plants survive in forested islands or fragments of wooded parkland—the patchy remnants of a once large and contiguous temperate forest ecosystem. Forest Park, in Queens County, is the largest of the urban woodlands on western Long Island, and it contains a sizeable portion of the local flora (Greller et al., 1979; New York City Department of Parks & Recreation [DPR], 1990, 1991). Early floristic inventories of Queens County and its environs have been critical to documenting not only local plant diversity but also changes in plant communities brought about by increased land development and human-induced disturbances (Greller, 1979; Greller, 1985; Greller, Panuccio & Durando, 1991; Harper, 1917a, 1917b). Despite Forest Park's size and status, it has not been closely studied, and thus little information is available to parkland personnel and administrators wishing to develop ecology-based management tools.
Knowledge of the floristic composition and structure of woodland communities is critical to understanding the greater dynamics of woodland ecosystems, and it is only with hard ecological data that sound management practices can eventually be applied. Currently, most of the fragmented woodland ecosystems within the city of New York have not been fully investigated beyond their floristic composition. The objective of this study—the first comprehensive one of the woodland since Greller, Calhoun, and Iglich (1979)—was to investigate the current health of the arborescent community in Forest Park.
History of Forest Park
In 1892, the New York legislature authorized the Brooklyn Parks Department to purchase the first parcel of parkland in Queens County (Figure 1). Additional acquisitions occurred into 1898, resulting in the expansion of the parkland to 218 hectares. Originally called Brooklyn Forest Park, it was transferred to the city of New York with the consolidation of Greater New York in 1898. The park, along with other parks in Brooklyn and Queens, was managed by the Brooklyn Parks Department until the Queens Department of Parks was established in 1911. Brooklyn Forest Park was renamed Forest Park and served as a multiuse park intended to provide a variety of recreational amenities to the public, including natural areas. Land use within the park area from the colonial period to the end of the 19th century had consisted mainly of timber harvesting, farming, and cattle grazing. These activities were halted when the park was established (New York City DPR, 1990). The designation of a 218-hectare park in Queens County amid a burgeoning human population (now exceeding 2.3 million inhabitants) was a crucial step in the conservation of local biodiversity.
In 1990, the New York City DPR's Urban Forest Education Program (UFEP) prepared a management plan for all urban forests within New York City. The major goals were to mitigate the impact of human disturbance on the ecology of park woodlands and to maintain and preserve native forest plant communities that were no longer subject to forces of natural disturbance. Forest Park became the first wooded parkland in Queens County evaluated for a natural-areas management plan by the New York City DPR's Natural Resources Group (NRG) (New York City DPR, 1996). This management plan has served as a model for all DPR wooded parks, such as neighboring Cunningham Park and Alley Pond Park (Tim Wenskus, NRG, personal communication, 2001). The plan identified vital plant communities and set priorities for woodland conservation. It also highlighted the park as containing the most extensive undisturbed forests in all of Queens County. Of the 218 hectares of parkland included in the management plan, an estimated 76% (167 hectares) was listed as closed forest canopy. The management plan, however, lacked an important ingredient for the management of the wooded landscape—a quantitative woodland census.
Location, Structure, and Condition
Forest Park (42° 30' north latitude and 73° 51' west longitude) is located in southwest Queens County and situated along the Harbor Hill terminal moraine of the southern point of the Glaciated Appalachian Plateau, formed by the Wisconsin glaciation (Cunningham & Ciolkosz, 1984; Greller et al., 1979; Sanders, 1974). The topographic elevations from the 1935 New York City Department of Parks maps series range from 18 to 42 meters (m) above sea level (Figure 2).
The woodland is mature throughout, as evidenced by the presence of large oaks, hickory, and flowering dogwood (Figure 3). Tree falls are common. Referencing the documented woody plant diversity in Forest Park and elsewhere in Queens County, Greller, Calhoun, and Iglich (1979) described the woodland as an oak, mixed dicot–dogwood type. However, visual observation suggests that the woodland is an oak-hickory-dogwood forest.
The overall knob-and-kettle topography is well vegetated with both herbaceous and woody flora. (In 2000, to account for the diversity of the understory flora within the study location, I conducted a survey of spring ephemerals and vines not included in the larger woodland census [Glaeser, unpublished data]. The survey revealed 15 families of herbs and ferns, represented by 26 genera; woody vines consisted of one family represented by two genera). Forest gaps atop the knobs are often covered by a mix of understory shrubs, saplings, grasses, and other herbaceous plants. In contrast to other neighboring wooded parks, most kettles in Forest Park lack seasonal water and are variably vegetated.
Click image to enlarge

Figure 3: A mature and aging Quercus velutina in the Northern Forest Management Zone of Forest Park surrounded by a high density and frequency of pioneer species such as the nonnative invasive Phellodendron amurense (saplings in the foreground) and Betula lenta (poles in the background).
From informal observations made throughout Forest Park, it is evident that unregulated high-impact activities such as mountain biking, horseback riding, and off-trail pedestrian use of the park have negatively impacted the plant community. Vandalism is equally evident in the form of cut trees, brush fires, graffiti, and litter. Though unquantified, the loss of plant cover and severe compaction and erosion of soil due to human activities has resulted in a degraded landscape in great need of restoration.
Methods
During the winter of 1999–2000, I delineated and surveyed a 50 × 100 m (0.5-hectare) permanent plot, divided into fifty 10 × 10 m quadrats, located within the 29-hectare Northern Forest Management Zone in Forest Park (Figure 2). A major criterion for plot selection was that no landscape-management activity—for example, thinning, tree planting, or weed control—was to have occurred within the study area. (All prior landscaping activity in the park was recorded in DPR woodland-management records.) This was to ensure that human-induced disturbance would not skew the data. The study plot, by general appearance, was representative of the greater Forest Park woodland.
All woody stems ≥ 2.0 centimeters (cm) diameter at breast height (DBH) were counted, regardless of tree or shrub characteristic. This was an unconventional DBH measurement, contrasting with those of forest censuses performed elsewhere within the New York City park system, in which only stems at least ≥ 7.6 cm DBH were counted (Greller et al., 1979; Rudnicky & McDonnell, 1989; Stalter, 1981; Stalter, Munir, Lamont & Kincaid, 2001). Each taxon within the plot was identified to species, and the botanical nomenclature followed Gleason and Cronquist (1991). Species importance values and family importance values were used to determine the dominance hierarchy or ranking of the woody taxa within the plant community (Ferreira & Prance, 1998; Mori, Boom, de Cavalino & dos Santos, 1983). Both measures of importance value (IV) were calculated as follows: IV = (relative density + relative frequency + relative dominance) × 100.
Click image to enlarge
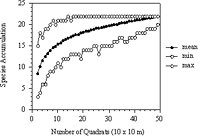
Figure 4: Randomization species-area curve generated from sampling quadrat combinations (NS=500) without replacement in Forest Park. The lower curve represents the minimum number of species and the upper curve the maximum number of species attained for each combination of quadrats.
A species-area curve (the accumulation of tree species as a function of the sample area) was prepared by approximate randomization analysis (Figure 4) (Manly, 1997; Mori, Becker & Kincaid, 2001; Rice & Kelting, 1955). Randomization shuffled the plot combinations 500 times without replacement.
Bootstrapping for confidence intervals of importance values at 95% was applied to the top ecologically dominant taxa. Confidence intervals were needed to measure the uncertainty of a sample statistic, such as importance values across the larger Forest Park plant communities (Dixon, 1993; Manly, 1997; Sokal & Rohlf, 1995). Frequency distribution for stem diameter, regression tests, descriptive statistics, and quartiles for diameter size classes were performed with StatView software (version 5.0, SAS, 1992). Upper and lower quartiles (25%) of the dataset were used to divide stems into the three stem-size classes. This approach was in contrast to other studies that utilized nonstatistical methods for determining size classes (Auclair & Cottam, 1971; Brewer, 2001; Parker, Leopold & Eichenberger, 1985).
Results
A total of 771 stems were identified, consisting of 22 tree and shrub species in 15 genera and 13 families. The mean number of species per quadrat was 5.60, and the mean number of stems per quadrat was 15.48. The stem density for all woody taxa measured at ≥ 2.0 cm DBH was 1,542 stems per hectare. In contrast, when calculated using a 10-cm DBH cut point, the mean stem density was 270 stems per hectare. This was a similar result to that found for neighboring Cunningham Park woodland (Queens County), which at the 10.0 cm DBH cut point had a stem density of 244 stems per hectare (Glaeser, unpublished data), and for the Alley Park woodland (Queens County), which had a density of 245.5 stem per hectare (Loeb, 1992). Quantitative measurements of diversity were as follows: Shannon-Wiener index (H') = 2.176; Simpson's index = 0.162.
The middle curve of the species-area curve shows the mean number of species per plot. The mean markedly increases and does not level off at quadrat 50, suggesting that censusing a larger area would reveal more taxa (Figure 4). The lower and upper curves are the minimum and maximum number of species found in the randomization of the quadrats, respectively. The maximum values on the curve level off at quadrat 15; it can be inferred from this that a maximum number of species (22 taxa) would be found in 15 quadrats.
Species Importance Values
Click image to enlarge
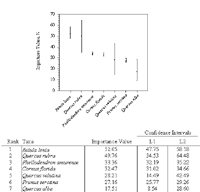
Figure 5: Importance values of seven ecologically dominant taxa in decreasing order of importance with 95% bootstrap confidence intervals. Bootstrap samples with replacement occurred for the 771 stems (NS=10,000). L1 and L2 represent the lower and upper limits of the confidence intervals.
Betula lenta, sweet birch (IV = 51.99), was the ecologically dominant species in the 0.5 hectare plot (Table 1). It had the highest relative density of all taxa, at 28.15%, a relative frequency of 14.18%, and relative dominance—an extrapolation of basal area—of 9.66%. The second-ranked species was Quercus rubra, northern red oak (IV = 49.55), which had a relative density of 4.28%, relative frequency of 8.16%, and relative dominance of 37.11%. The third-ranked species was the nonnative invasive Phellodendron amurense, Amur corktree (IV= 33.35) (see Glaeser & Kincaid, 2005), which had a relative frequency of 9.93% and relative dominance of 2.92%. Note that at 20.49%, this species' relative density was second to that of Betula lenta. The fourth species in the dominance ranking was Cornus florida, flowering dogwood (IV = 32.45). This understory tree ranked third in terms of relative density (14.92%). The dominance ranking and abundance of C. florida is of interest because of its susceptibility to numerous foliage pathogens such as anthracnose (Discula species). Quercus velutina, black oak (IV= 28.07), ranked fifth in overall ecological dominance; however it ranked tenth in relative density (1.30%). Prunus serotina, black cherry (IV = 27.14), ranked sixth in ecological dominance; it had a relative density of 11.0% and was the third most frequently encountered tree species, with a relative frequency of 12.77%. Quercus alba, white oak (IV=17.44), ranked seventh in ecological dominance but third behind Q. rubra and Q. velutina in relative dominance at 12.21%. Acer rubrum L. (red maple), A. platanoides L. (Norway maple), Liriodendron tulipifera L. (tulip tree), Ilex verticillata L., A. Gray (common winterberry), and Nyssa sylvatica Marshall (black gum) appeared as singletons and ranked low in ecological dominance due to low counts and small diameter size.
The bootstrap 95% confidence intervals were used to determine the certainty of a parametric mean, such as the species importance values (Manly, 1997). Confidence intervals were determined for seven of the ecologically dominant taxa (Figure 5).
Family Importance Values
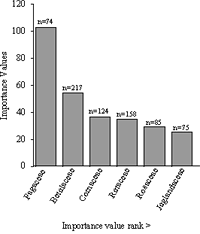
Family importance values were applied to the 13 tree and shrub families (Table 2). The Fagaceae was the dominant and richest of the tree families. It was represented by five species and collectively contained 74 individual stems comprised of Quercus rubra, Q. velutina, Q. alba, Castanea dentata, and Fagus grandifolia. The collective IV for species within the Fagaceae was 102.57 out of a possible 300. The relative density was a low 9.60%, while the relative frequency was 16.05%, or equivalent to that of the top three ranking families. The Fagaceae had a relative dominance of 77.5% and a combined basal area of 11.71 square meters, which was eight times the basal area of the next-dominant-ranking family (Betulaceae). Members of the Fagaceae were found in 39 of the 50 sampled quadrats (78.0%). The second-ranked family in the dominance hierarchy was Betulaceae (IV= 54.27), represented by a single species, Betula lenta. Owing to this species's abundance, Betulaceae had a relative density of 28.03% and relative frequency of 16.46%. The third-ranked family was the Cornaceae (IV=36.02), represented by two species: Cornus florida and C. alternifolia L.f. Both the relative frequency and relative density of this family were 16% (placing it very close in relative frequency to Fagaceae and Betulaceae); however, its relative dominance, at 3.40%, was markedly low. Fourth in family ranking was the Rutaceae (IV=34.94), represented by Phellodendron amurense. The Amur corktree had a relative density of 20.49%, second highest overall next to the Betulaceae (Figure 6).
Stem Diameters
The use of lower and upper quartiles (or the 25 percentile) of the sampled population statistically partitioned all arborescent stems into small- and large-size diameter classes and a central 50-percentile for the midsize-diameter class. The diameter-size classes were as follows: small-size diameters (2.0 to < 2.8 cm DBH, n = 202); midsize diameters (2.8 to < 7.48 cm DBH, n=372); and large-size diameters (7.48 to 116.7 cm DBH, n=197).
Species richness within the small-size-diameter class was 19 tree species, representing 13 families. Stem density was 402 stems per hectare, and the combined basal area (BA) was 0.893 square meters. The top four ecologically dominant taxa, in decreasing order of importance, were Betula lenta (IV=73.54), Phellodendron amurense (IV= 65.45), Cornus florida (IV= 44.99), and Prunus serotina (IV= 36.44) (Table 3). Betula lenta displayed the greatest relative density with 26.8%, followed by Phellodendron amurense (24.38%), Cornus florida (14.43%), and Prunus serotina (11.94%). The most frequent taxon encountered was Betula lenta (relative frequency 20.18%), and it was followed by Phellodendron amurense (16.67%), Cornus florida (15.79%), and Prunus serotina (12.28%).
The midsize-diameter class contained the most abundant stems of the three size classes: n=372 (Table 4). Species richness within this group was 17 tree species, distributed among 10 families. Stem density was 744 stems per hectare, and basal area (BA) was 6.66 square meters. Within this size class, the largest tree was Cornus florida (7.48 cm DBH). The top four ecologically dominant taxa were, in decreasing order of importance, Betula lenta (IV=81.68), Phellodendron amurense (IV=61.47), Cornus florida (IV=48.92), and Prunus serotina (IV=30.62). The most frequently encountered taxon was Betula lenta (relative frequency 18.59%), and it was followed by Phellodendron amurense (14.74%), Cornus florida (17.95%), and Prunus serotina (11.54%). Betula lenta also displayed the greatest relative density (31.45%), and it was followed by Phellodendron amurense (24.19%), Cornus florida (14.79%), and Prunus serotina (9.68%). Betula lenta ranked highest in relative dominance (31.64%) and was followed by Phellodendron amurense (22.53%), Cornus florida (16.19%), and Prunus serotina (9.40%).
The species richness within the large-size class was 13 tree species, distributed among 7 families. Stem density was 394 stems per hectare, and basal area (BA) was 145.48 square meters. The ecologically dominant taxa within this group were, in decreasing order of importance, Quercus rubra (IV= 70.25), Betula lenta (IV= 51.11), Quercus velutina (IV= 34.84), and Cornus florida (IV= 33.34) (Table 5). Unique among the dominant taxa in this size class is Betula lenta; though second in ecological dominance, it had a high relative density (23.35%) compared with Cornus florida (15.74%), Quercus rubra (14.72%), and Prunus serotina (12.69%). The most frequently encountered taxon was Betula lenta, with a relative frequency of 19.15%, and it was followed by Quercus rubra (16.31%), Cornus florida (14.89%), and Prunus serotina (12.77%). The oaks—Quercus rubra, Q. alba, and Q. velutina—displayed the greatest relative dominance in the large-size class. The largest oak specimen was a Quercus rubra measuring 116.7 cm DBH. Though the Quercus species were low in abundance, their larger basal areas accounted for the increased relative dominance.
The basic structural characteristics of the top three ecologically dominant taxa within all the diameter-size classes were compared (Table 6). Betula lenta was within the top three taxa in all size classes. It was the dominant taxon within the small- and midsize-diameter classes and ranked second to Quercus rubra within the large-size class. Cornus florida was among the top three taxa in the small- and midsize classes. The nonnative invasive Phellodendron amurense ranked second in dominance within the small-size and midsize classes. Throughout the study plot, the largest trees were the oaks, yet they only made up 9% of the entire sampled population.
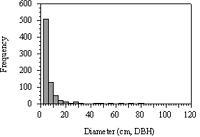
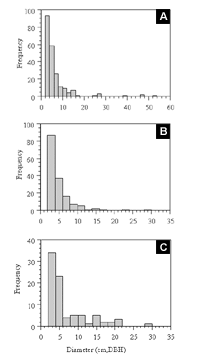
The frequency distribution for all tree diameters placed 66% of all stems (n=771) within the first 2.0–4.0 cm histogram interval (Figure 7). Betula lenta, Phellodendron amurense, Cornus florida, and Prunus serotina composed 80% of the stems within the first histogram interval and 70% in the second histogram interval.
Discussion
The 0.5-hectare study plot contained a rich array of trees and shrubs with ≥ 2.0 cm DBH. This low DBH cut point allowed for the inclusion of many more species and stem counts than would a cut point of ≥ 7.6 cm DBH, a measurement used in previous wooded parkland inventories (Greller et al., 1979; Stalter, 1981). Twenty-two species were tallied from sampling 771 stems at ≥ 2.0 cm DBH. A notable fact is that the 22 species were identified within the 2.0–7.6 cm DBH range (n=580), which is 9 more species than would have been identified had the DBH cut point been ≥ 7.6 cm. Of the 22 species identified in the Forest Park woodland, 19 were native to the temperate Northeast, and three were nonnative invasive species (Phellodendron amurense, Acer platanoides, and Rhamnus frangula [glossy buckthorn]).
The 95% confidence intervals applied to species importance values for the top seven dominant taxa provided an indication of confidence in these values. The taxa with the larger sample sizes (n)—for example, Betula lenta (n=217), Phellodendron amurense (n=158), Cornus florida (n=115), and Prunus serotina (n=85) had a smaller confidence interval, and thus I am more confident that the true values lies within the range of the limits (Figure 5). Based on the overlapping confidence limits of the seven ranked taxa and on inferential ecological dominance for the New York City urban woodland at large, the dominance ranking of Quercus velutina, for example, may be from rank number two to rank number seven. For Phellodendron amurense, it may be from rank number two to rank number five.
It is theorized that forest disturbances (of any type and scale) result in gaps that are heterogeneous due to gap-phase regeneration. Recovery from disturbances often results in a mosaic of forest patches at different stages of succession. Trees found within gaps may consist of either pioneer species or climax species or both, and thus gap-phase regeneration adds to stand diversity. Numerous studies have related forest composition to the size and frequency of these disturbances (Brokaw & Scheiner, 1989; Veblen, 1989; Whitmore, 1989). In general, plant communities respond to disturbances differently, and their responses vary with the type of disturbance, be it logging (Ramirez, 2001; Yoshida, Yoko, Ozawa, Mahoko & Shibata, 2005), anthropogenic pressure, fires (Loeb, 2001), or natural tree falls, such as those observed in Forest Park. I believe that a combination of tree falls, herbivory, and other unquantified disturbances has promoted a tree species distribution and composition more typical of pioneer species than of climax species in this mature oak-hickory hardwood forest.
Of the top 12 ecologically dominant trees and shrubs in Forest Park, 3 share characteristics associated with pioneers of disturbed sites. Pioneer trees generally produce copious amounts of small, readily dispersed seeds; have seeds that can only germinate in full sun; and are relatively short-lived (Whitmore, 1989). Betula lenta, Phellodendron amurense, and Prunus serotina possess some or all of these traits and are of special interest because of their high representation within the study plot (density and frequency) (Figure 8). The pioneer status of these species is supported by their high representation within the small and mid-size diameter classes. The environmental variables influencing the demographic responses of these pioneer taxa are undetermined for Forest Park. Yet previous reporting on two other Betula species in Japan has indicated that increased light intensity—affecting such variables as soil properties, litter accumulation, canopy cover, and snow depth—is the most important factor for Betula species dominance (Yoshida et al., 2005). Yoshida suggested that the presence of any canopy plays an important role in the distribution success of Phellodendron amurense due to the fact that the species' seeds are bird-dispersed. Seedling photosynthetic performance under shade conditions is also a factor.
Both Betula species and Phellodendron amurense are regarded as shade intolerant in the forests of Hokkaido, Japan (Koike & Sakagami, 1985; Yoshida & Kamitani, 1999). The environmental variables that influence the success of these pioneer taxa in the forests of northern Japan may be considered with regard to the situation in Forest Park, at least until further empirical investigation occurs.
The distribution of woody stems in the Forest Park study site was typical for a mature woodland stand in that it contained an abundance of small- to mid-size-diameter stems and relatively few large stems (Figure 7). This skewing of the stem-diameter distribution toward the early stages of gap-phase regeneration is widely accepted as a general trend for mature and aging forest (Hara, 1988). That pioneer taxa were highly represented in this study is also typical, as Hara suggested. However, though the Fagaceae was the ecologically dominant family within the large-diameter size class, it had very little representation within the small- and mid-size-diameter classes (Table 3 and Table 4). The current cause of the depauparate regenerative capacity among this family is speculative, yet the consequences are a concern for the greater ecology of Forest Park.
Predation upon seed resources by overabundant populations of Sciurus carolinensis Gmelin (eastern gray squirrel), Tamias striatus L. (eastern chipmunk), and Mus species (common field mouse) may be occurring. It is possible that representatives of the Fagaceae may be found in a size-class measurement of ≤ 2.0 cm DBH or as newly emerged seedlings. A recensusing of the Forest Park woodland scheduled for 2010 may register additional Fagaceae saplings that have grown into the ≥2.0 cm DBH size class.
It has long been established that nonnative invasive species are a threat to native ecosystems. Invasive species impact upon all levels of biotic organization by modifying the fundamental properties of ecosystems (Henderson, Dawson & Whittacker, 2006). Invasive species in eastern U.S. forests may out-compete natives, occupy unfilled niches, or have negative allelopathic impacts on the growth of their arborescent neighbors. Threats to the diversity of native plant populations by the establishment of nonnative plants have been noted elsewhere in Queens County (Stalter, Munir, Lamont & Kincaid, 2001). It has recently been proposed that the nonnative invasive Phellodendron amurense (with a relative density of 20.49% in this study) may be interfering with the growth of the Fagaceae. North of Philadelphia (Montgomery County, Pennsylvania) as well as in the New York City area (Queens and Bronx County), P. amurense has aggressively invaded disturbed forests. Due to lack of regeneration of native species, oak-hickory hardwood forests are being transformed into Phellodendron forests (The Nature Conservancy, 2005). It has been suggested that root exudates from Phellodendron amurense may be inhibiting the growth of its neighbors in oak-hickory forests.
This census revealed an extremely low regenerative potential for all the oak and other traditional canopy trees amid highly abundant pioneers and a successfully colonizing nonnative invasive tree, Phellodendron amurense. Considering that the 0.5-hectare study plot is representative of the greater Forest Park, the lack of regeneration of the canopy trees—and the potential loss or disruption of their contribution to the ecology, habitat, and microclimate dynamics of the forest—is a cause for serious alarm.