Microhabitat Selection and Singing Behavior Patterns of Male House Finches (Carpodacus mexicanus) in Urban Parks in a Heavily Urbanized Landscape in the Western U.S.
by Esteban Fernández-Juricic, Rachael Poston, Karin De Collibus, Timothy Morgan, Bret Bastain, Cyndi Martin, Kacy Jones, and Ronald Treminio
Department of Biological Sciences, California State University, Long Beach, 1250 Bellflower Blvd., Long Beach, CA 90840-3702.
Abstract
We assessed the role of park size, habitat structure, human disturbance (pedestrian rate and ambient noise), and the number of conspecifics in the distribution, spacing, and singing behavior of male house finches (Carpodacus mexicanus) in urban parks in southern Los Angeles County and north Orange County, California. We found that the probability of house finch males occupying urban parks increased with park size and tree structure (total tree cover, tree height, and the number of stems 30 to 50 centimeters in diameter)—two features that may increase the availability of suitable nesting substrates. Nearest neighbor distance between singing males increased with denser vegetation structure (e.g., number of stems), probably because of better nesting and foraging resources or greater availability of protective cover, which would reduce aggregation. Males increased their singing rates in the most exposed parts of their perches (upper and outer portions). They also raised the low frequency of their songs to reduce the masking effects of high ambient noise levels. However, the number of notes per song decreased with ambient noise, and since females are attracted to long songs, this could decrease mating opportunities. Our results point out some of the mechanisms house finch males use to increase their breeding success in urbanized areas and suggest that this success may vary depending on the specific spatial location of nesting areas within a city.
Key words: ambient noise, birds, distribution, males, spacing behavior, singing rate, songs, urban ecology, urban parks
Introduction
Urban sprawl has modified natural landscapes by changing the availability and configuration of suitable habitat for wildlife and by altering the nature and frequency of human-wildlife interactions (Fernández-Juricic & Jokimäki, 2001; Marzluff, 2001). The size of remnant habitat fragments is a primary influence on the number of birds that urban habitats can sustain (Fernández-Juricic, 2000; Crooks, Suarez & Bolger, 2004), mainly because of the area requirements of interior, rare, and low-abundant bird species (Crooks, Suarez, Bolger & Soulé, 2001; Fernández-Juricic, 2002). Suitable fragments might be urban parks or natural areas surrounded by urban development. Vegetation structure has also been found to increase species richness by enhancing key habitat resources that facilitate the colonization of certain species (Donnelly & Marzluff, 2004; Feldman & Krannitz, 2004; White, Antos, Fitzsimons & Palmer, 2005).
High levels of human visitation to these urban fragments may reduce the spatial and temporal access certain birds have to suitable resources and decrease the chances of park occupation by low-disturbance-tolerant species (Fernández-Juricic, 2002). Furthermore, human-generated ambient noise (car traffic, trains, airplanes, industries, etc.) can mask the communication systems of some birds (Rabin & Greene, 2002; but see Leader, Wright & Yom-Yov, 2005), causing them to adjust the vocal structure of their songs (Slabbekoorn & Peet, 2003; Brumm, 2004). Taken together, this evidence suggests that urban habitats are complex environments and that different species use various habitat-selection mechanisms for breeding purposes (Sedlacek, Fuchs & Exnerova, 2004).
So far, researchers have mainly concerned themselves with minimizing the decline of interior bird species in urban landscapes, because these species appear to be more sensitive to urbanization (Savard, Clergeau & Mennechez, 2000; Fernández-Juricic & Jokimäki, 2001; Marzluff & Ewing, 2001; Chace & Walsh, in press). However, edge species, many of which have thrived in urban areas around the world (Marzluff, 2001), make interesting models for understanding the life-history traits and ecological factors that enable some birds to adapt successfully to human-dominated landscapes. We studied one such species, the house finch (Carpodacus mexicanus), which is considered native to the western U.S. but has spread throughout the eastern portion of North America. It inhabits open and semiopen areas, particularly in urban and suburban locations (Hill, 1993, 2002).
Our goal was to assess the role of fragment size, habitat structure, human disturbance (pedestrian rate and ambient noise), and number of conspecifics in the distribution, spacing, and singing behavior of male house finches in urban parks. We focused on the birds during the breeding season to understand the potential mechanisms influencing mating opportunities. Male house finches display bright carotenoid-based plumage coloration (Hill, 2002) and emit a song comprised of a series of notes, sometimes followed by a trill and a buzz (Bitterbaum & Baptista, 1979; Hill 1993). Male songs appear to be more involved in female attraction than in male-male competition due to the apparent lack of territoriality in this species (Thompson, 1960; Hill, 1993, 2002; Nolan & Hill, 2004). However, little is known about the effects of male competition on spacing and singing behavior of house finches (Hill, 1993).
Specifically, we studied (a) the effects of park size, vegetation structure, and pedestrian rate on the occurrence of male house finches in urban parks; (b) the influence of the number of singing males and tree structure (as indicators of competition and the availability of suitable vegetation) on the spacing behavior of males; (c) the effect of the number of males and degree of exposure in perches on their singing rate (number of songs per unit time); and (d) the role of ambient noise on the frequency and duration of male songs.
Methods
Study Area
We studied the house finch (Carpodacus mexicanus) in its native California, where no geographic variations in vocalization are reported to exist (Bitterbaum & Baptista, 1979). (In other parts of the house finch's distributional range, different dialects have been recorded; see Pytte, 1997, and Tracy & Baker, 1999).
The study was conducted at several sites in south Los Angeles County and north Orange County during the spring of 2005, mostly in the city of Long Beach. We chose 35 parks (Table 1) that were representative of the variability in size of the parks in the region. All had wooded areas with tree cover, introduced and native shrub species, and areas of watered grass.
Male House Finch Surveys
Each park was surveyed two or three times during the spring of 2005, on weekday mornings from 6:00 a.m. to 9:30 a.m. We recorded the presence and number of male house finches and surveyed male singing and courtship behavior. In parks greater than two hectares in size (23 of the 35 parks), we set up 100-by-50-meter transects in both the interior and at the edges of parks, each separated by 100 to 200 meters (Järvinen & Väisänen, 1977). Prior to beginning the surveys, observers were trained to visually estimate 25 meters on each side of the transect central path with less than 10% error. The same transects were used in each visit. The number of transects per wooded park was established in proportion with a logarithmic scale of the size of each park. In each of the parks less than two hectares in size (12 parks), we sampled the whole park area for a period of time that was proportional to the time used in sampling the line transects (see more details of the survey techniques in Fernández-Juricic, 2000, 2004).
Three independent factors were considered: park size, vegetation structure, and human disturbance. Park sizes were derived from the city websites of Long Beach and Seal Beach.
We measured vegetation-structure traits in 25-meter-radius circular plots distributed at 30-meter intervals along transects in parks larger than two hectares, and randomly in parks less than two hectares (see also Fernández-Juricic, 2000). The number of plots per park was determined by park size (log-transformed). We recorded the following: cement cover; grass cover; bare-ground cover; bush cover; total tree cover; coniferous tree cover; deciduous tree cover; mean tree height; mean bush height; number of tree species; number of bush species; and number of tree stems in four diameter-at-breast-height (dbh) ranges (< 10 centimeters dbh, 10–30 centimeters dbh, 30–50 centimeters dbh, and > 50 centimeters dbh). Cover variables were visually estimated in percentages following Prodon and Lebreton (1981) and corresponded to different vegetation substrates. Tree-cover measurements were based on the area of the overstory. The number of stems was determined by counting stems in each size category in the sampling plots. Tree and bush heights were estimated with a pencil by visually rotating the plant tips 90 degrees onto the ground and then measuring the ground distance with a meter tape (± 0.05 meters). Values for each vegetation trait measured at each transect were averaged for each whole park.
We also recorded the number of pedestrians (walking and sitting) in the morning (7:00 a.m. to 12:00 p.m.) and in the afternoon (12:01 p.m. to 7:00 p.m.) in five-minute periods within 50-by-50-meter plots placed randomly inside the bird transects (one plot per transect). Measurements were conducted twice at each park (once during a weekday and once on the weekend). Final figures were averaged over the two visits and transformed into mean numbers of pedestrians/5 minutes/10 hectares per park (see also Fernández-Juricic, 2004).
Spacing Behavior
During the surveys, we also mapped the location of male house finches singing in each park inside and in between transects to estimate nearest neighbor distances (the distance between a male and its closest neighbor), following Krebs (1998) and Forsman, Mönkkönen, Inkeröinen & Reunanen (1998). Mapping was done using a handheld GPS device and visual landmarks. Distances were calculated using ArcView GIS software (version 3.3) and corroborated with a web-based distance calculator available online at www.wcrl.ars.usda.gov/cec/java/lat-long.htm. We estimated neighbor distances within a particular visit and then calculated the mean nearest neighbor distances over all visits per park. We included in the analysis the mean neighbor distances of house finch males in only 14 of the 35 urban parks (one mean value per park), because (a) house finches were not detected at all the parks, and (b) we only calculated neighbor distances when at least two house finches were present in the same sampling day in a park (thus avoiding temporal biases in the GPS position estimates). We also counted the number of neighbor males around the focal male in a 50-meter radius, as the density of individuals could affect patterns of spacing behavior (Krebs, 1998). As explanatory variables, we took into account the habitat structure factors that significantly explained the probabilities of park occupation: total tree cover, mean tree height, and number of stems < 10 centimeters dbh, 10–30 centimeters dbh, and 30–50 centimeters dbh.
Male Singing Rates
We restricted our study to males singing from perches and did not consider those cases in which males sang flying (Hill, 1993). We recorded singing rates from early March to early May 2005, between 5:45 a.m. and 9:00 a.m. on days without rain or wind. Upon finding a singing male within a transect, we recorded his songs with a Sony TCM-200DV portable tape recorder and calculated the number of songs emitted per minute. We only considered recording samples with durations of at least 90 seconds and up to a maximum of 20 minutes; recording was stopped when birds left their perching trees. We included in the analyses the singing rates of 68 males (one value per male) from 17 urban parks. Singing rates were recorded only once for a given transect to minimize the probability of resampling males. For each male, we recorded the number of neighbor males singing (which was equivalent to the number of singing males in the transect), the type of perch (tree, bush, fence, or power line, etc.), portion of the tree or bush (inner, outer) on which the animal was perching (if it was perching in a tree or bush), time of year, time of day, and temperature. Since time of day and temperature were highly correlated (Pearson correlation, r = 0.57, P < 0.001), we elected to include only the latter in the analysis. We also recorded the height of the perching bird and that of the vegetation substrate, as described before, and divided them to estimate the perch-height ratio. Ratio values close to 1 indicated that a male was perched relatively high and exposed in the substrate.
Song Structure
Song recordings to assess house finch vocal structure were recorded in the same parks, but on different days to those on which singing rates were recorded. We used an Audio-Technica AT815b line/gradient condenser microphone to record onto a Sony portable minidisc recorder (M2-N10). Recordings were taken from early March to early May, in the 2004 and 2005 breeding seasons, between 5:45 a.m. and 9:00 a.m. on days without rain or wind. The recording level of the minidisc recorder was the same at all sites. We could not mark the males, but to minimize the chances of recording the same individual more than once, we only visited each park once, and within each park we only recorded individuals that were separated by at least 100 meters. To minimize changes in song amplitude with distance, house finch males were recorded from a distance of 6 to 7 meters (microphone to perch). The microphone was held steadily in the most direct line toward the singing male. To reduce attenuation of songs by physical barriers, only males within unobstructed view were recorded. However, there are two sources of bias in our recordings: (a) the 1-meter difference in recording distance could generate variations in amplitude up to 1.3 dB, and (b) the orientation of the bird in relation to the microphone was not recorded, though this could also affect song amplitude.
We recorded the bird songs until 15 minutes had lapsed, the male stopped singing for more than 5 minutes, or until it flew away. We recorded 5 to 20 songs per male, with a total of 44 males in 16 urban parks. We also recorded time of year, time of day, and temperature as potential confounding factors. Time of the day and temperature were less strongly correlated than in the singing-rate samples (Pearson correlation, r = 0.19, P < 0.05), so we decided to include both in the analysis. We also established whether or not other males were singing while we recorded focal males, as birds can vary vocal structure in response to the presence of conspecifics (Brumm & Todt, 2002; Cynx & Gell, 2004).
Male songs were digitized at 22 kHz and 16 bits and analyzed using Raven 1.2 software (Charif, Clark & Fisrup, 2004). Over each song, we measured the following in a 22 kHz range: low frequency (Hz), frequency range (Hz), number of notes, and duration (ms). Number of notes was positively correlated with song duration (Pearson correlation, r = 0.95, P < 0.001), so we presented the results of the former. We averaged all these vocal parameters for each male across his songs, so that each data point in the analysis corresponded to a different male.
While recording a male, we also recorded 5 to 10 minutes of ambient noise before, in-between, and after song bouts. Within each male's recording session, we digitized ten randomly selected 30-second segments in which the male was not singing at 22 kHz and 16 bits, recorded ambient RMS amplitude with Raven 1.2, and calculated mean values for each session in a 22 kHz range. RMS amplitude is the sum of the squared values of amplitude for a sound (Charif et al., 2004). RMS amplitude takes into account minimum and maximum amplitude values (Bradbury & Vehrencamp, 1998) and was measured with Raven 1.2 in micro Pascals (µPa) (H. Mills, personal communication, October, 2005). A similar procedure has been recently used to record and estimate ambient noise (Leonard & Horn, 2005).
Statistical Analysis
Throughout the statistical analyses, logarithmic transformation was performed on certain variables (park area, pedestrian rate, nearest neighbor distance, number of stems < 10 centimeters dbh, number of stems of 30–50 centimeters dbh, number of songs per minute), and arcsin transformation was performed on another (perch-height ratio) to meet normality and homogeneity of variance assumptions.
To reduce the number of vegetation variables on the habitat structure data per park, we performed a principal component analysis (PCA) on the correlation matrix. Only those PCA factors with eigenvalues > 1 were selected (Kaiser criterion), and factor loadings were rotated with a varimax raw transformation.
To analyze the effects of park area, pedestrian rate, and habitat structure (PC1–PC6, see Results) on the probabilities of park occupation by house finches, we used a logistic regression with a binomial dependent variable (presence/absence) and a logit link function. Recent studies point out the relevance of using alternative model-selection criteria, such as information theoretic approaches, to model species distributions (Rushton, Ormerod & Kerby, 2004), particularly in multicausal scenarios (Stephens, Buskirk, Hayward & Del Rio, 2005). We then calculated the Akaike information criterion (AIC) of all combinations (256) of the eight independent variables studied, and chose the model with the lowest AIC value, following Burnham and Anderson (2002).
We used stepwise multiple regressions to assess the effects of total number of individuals, total tree cover, mean tree height, and number of stems (< 10 centimeters dbh, 10–30 centimeters dbh, 30–50 centimeters dbh) on nearest neighbor distances. Both backward (F to enter = 11, F to remove = 10) and forward (F to enter = 2.5) selection procedures were performed to identify the most significant factors.
We modeled the variability in singing rates with general linear models (GLM), using two categorical and three continuous independent variables: number of singing neighbor males (1, 2, and > 3), type of perch (tree/bush, and artificial—fence/power line/building), perch-height ratio, time of year, and temperature. With a subset of these data, we conducted another GLM to assess the effects of portion of the tree in which the male was perching (inner, outer), controlling for the effects of the significant factor found in the previous analysis.
We used one-way ANOVA to determine whether the presence or absence of conspecifics singing could affect the frequency and temporal parameters of focal male songs. We also performed a GLM to assess the effects of ambient noise on low frequency, frequency range, and number of notes, while controlling for the potential confounding effects of time of year, time of day, and temperature.
Statistical analyses were conducted with SPSS 13.0 and Statistica 7.0 software.
Results
Habitat Structure Factors
The PCA identified six factors (PC1–PC6) with eigenvalues > 1, explaining 83.4% of the variability in park habitat structure (Table 2). PC1 was associated with ground cover—with positive values indicating relatively more grass, and negative values indicating relatively more bare ground cover. PC2 was associated with tree structure—with positive values characterizing parks with greater total tree cover, higher tree height, and more stems of 30–50 centimeters dbh. PC3 was positively associated with bush height, number of bush species, and number of stems > 50 centimeters dbh. PC4 was a tree composition axis—with positive values indicating greater deciduous tree cover and negative values indicating greater coniferous tree cover. PC5 was negatively associated with cement cover, and PC6 was positively associated with number of stems < 10 centimeters dbh and number of stems of 10–30 centimeters dbh.
Presence of House Finch Males in Urban Parks
Park size ranged from 0.09 to 122.94 hectares (mean ± SD, 11.80 ± 25.29). Only two of the independent factors were correlated: park area and PC4 (Pearson correlation, r = –0.35, P = 0.041). This means that more coniferous cover and less deciduous cover was associated with larger parks. All other correlations were nonsignificant (P > 0.236).
House finch males were found in 26 of the 35 parks studied. The logistic regression model accounting for the probabilities of park occupation with the lowest AIC (41.27) included two of the eight factors considered: park size and PC2 (B coefficients, intercept = – 1.47, park size = 1.81, PC2 = 0.90; χ² = 9.21, d.f. = 2, P = 0.010). Thus, the probability of house finch males being present in urban parks increased with the size of the park and with tree structure (total tree cover, tree height, and the number of stems 30–50 centimeters dbh).
Distance Between Singing Males
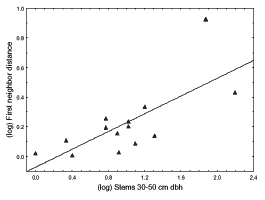
The mean (± SD) distance between male house finches within parks was 279.49 ± 374.53 meters. Neighbor distance within parks was affected by the number of stems 30–50 centimeters dbh (coefficients, intercept = – 0.073, stems 30–50 cm = 0.300; F 1,12 = 13.57, P = 0.003, Adjusted R² = 0.49; see Figure 1): Distance to the closest singing male increased with the number of stems of medium to large trees. This result was found in multiple regressions with both forward and backward selection procedures, which failed to include the other five factors studied: mean number of singing males in the park, tree height, total tree cover, and stems < 10 and of 10–30 centimeters dbh. A similar result was also found even after arbitrarily entering the number of singing males in the previous model (intercept, coefficient = 0.001, t11 = 0.01, P = 0.992; number of males, coefficient = –0.016, t11 = –0.79, P = 0.441; total stems 30–50 cm, coefficient = 0.296, t11 = 3.56, P = 0.004; F 2,11 = 6.89, P = 0.011, Adjusted R²= 0.48).
Male Singing Rates
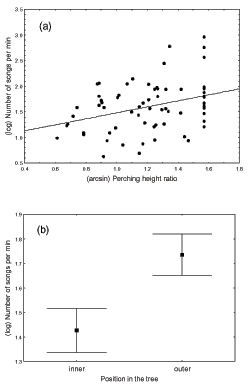
The mean (± SD) singing rate of house finch males across parks was 4.36 ± 3.07 songs per minute. The singing rate of male house finches was influenced by perch-height ratio: The higher the males were in the perching substrate, the more songs per minute they emitted (Table 3, Figure 2a). Number of neighboring males, type of perch, temperature, and time of year did not exert a significant influence (Table 3). A subset of these data was then used to assess the effects of singing position in the tree. We found that males increased the number of songs per minute while perching in the outer portions of the tree or bush (Figure 2b), controlling for the significant effects of perch-height ratio (Table 3).
Structure of Male Songs in Relation to Ambient Noise
The vocal parameters of the songs of the studied house finch males were characterized as follows (mean ± SD): low frequency, 1,720.98 ± 129.35 Hz; high frequency, 15,421.94 ± 1,432.91 Hz; frequency range, 15,421.94 ± 1,432.91 Hz; and number of notes per song, 20.79 ± 4.27. All our ambient noise recordings yielded a low frequency equal to 0 Hz. The mean high frequency of ambient noise was 19,750.46 ± 1,169.04 Hz, and the RMS amplitude was 1,734.32 ± 1,112.59 µPa. The two most important noise sources in our study area were car traffic and air traffic.
We first assessed whether the presence or absence of conspecifics would affect frequency and temporal vocal parameters of house finch songs. We found that none of these parameters was affected by the social context (low frequency, F 1,42 = 3.09, P = 0.086; frequency range, F 1,42 = 0.94, P = 0.338; number of notes, F 1,42 = 0.06, P = 0.803).
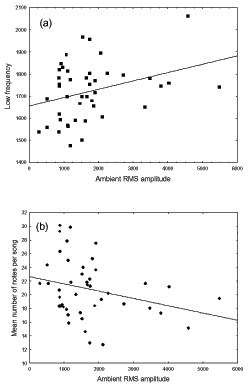
However, male house finches changed the frequency and temporal structure of their songs in relation to ambient noise. Controlling for the effects of time of the year, time of the day, and temperature, we found that house finch males increased the low frequency of their songs in areas with higher ambient noise (Table 4, Figure 3a). However, the frequency range of songs did not vary with ambient noise (Table 4). We also found changes in the temporal structure of songs: the number of notes per song decreased with increasing ambient noise (Table 4, Figure 3b).
Discussion
Our results show that male house finches select relatively large urban parks with high availability of medium- to large-size trees, increase their neighbor distances with an increase in the number of stems, increase singing rates in the most exposed parts of the perch (upper and outer portions), and change the low frequency and number of notes of their songs in relation to high ambient noise levels.
The higher chances of occupation in larger parks could be the result of the reported association between park area and coniferous cover (PC4). Although the house finch does not appear to be particularly associated with coniferous forests in the western part of its distributional range (Hill, 1993), it uses open coniferous forests at high elevations (Grinnell & Miller, 1944) and prefers to nest in conifers in Ontario (Graham, 1988). It seems that male house finches in our study area prefer large parks with greater coniferous cover, probably because the thicker coniferous vegetation provides better nesting substrates. Furthermore, singing house finch males occupy parks with taller and denser vegetation (i.e., a high availability of tree cover, tall trees, and a large number of stems 30–50 centimeters dbh). This might indicate a preference for nesting sites that are less vulnerable to predation and human disturbance and have a higher availability of insects to feed nestlings.
We also found that the number of neighbor males did not affect neighbor distance or singing rates—a confirmation of earlier reports that the singing behavior of this species is not greatly influenced by competitor presence (Thompson, 1960). However, the higher the availability of stems of 30–50 centimeters dbh, the greater the distance between neighbor singing males. Although this species does not defend large territories and can nest in loose colonies (Hill, 1993; 2002), our result could be interpreted in terms of better nesting and foraging resources found in areas with denser vegetation, as explained above. Alternatively, in parks with fewer stems 30–50 centimeters dbh, the perceived risk of predation might be higher due to reduced availability of protective cover, and house finches might be decreasing neighbor distance in order to dilute that risk (e.g., Forsman et al., 1998).
The increase in singing rates by males may be associated with higher mating probabilities. Previous studies show that female house finches show preference for more colorful males and males that emit songs at faster rates (Hill, 1990; Nolan & Hill, 2004), and that colorful males nest earlier (Hill, Nolan & Stoehr, 1999) and have higher nesting success (McGraw, Stoehr, Nolan & Hill, 2001). Singing rates increased in the most exposed portion of perches. When perching in trees, male house finches may display at higher areas in the trees if these areas are of better quality for breeding—thereby increasing their chances for reproductive success. Another interpretation, which particularly applies to artificial perches (fences, power lines, buildings), is that males may increase their visual and acoustic exposure to females by singing from perches that are more easily detected from the distance, as found in golden-winged warblers Vermivora chrysoptera (Rossell, 2001). However, being more exposed could also attract more predators, or it could increase the chances of early detection of a predator through improved antipredator vigilance (Krams, 2001). Future studies should establish the trade-offs between breeding success and predation risk for house finch males singing from exposed perches.
Another shortcoming of singing from exposed positions in urban areas is that house finch males may face greater acoustic disturbance. There are two types of variations to counteract the masking effects of higher noise levels, which are usually concentrated on low frequencies in cities: amplitude shifts (the Lombard effect; e.g., see Cynx, Lewis, Tavel & Tse, 1998; Manabe, Sadr & Dooling, 1998; Pytte, Rusch & Ficken, 2003; Kobayasi & Okanoya, 2003; Brumm, 2004), and frequency shifts (Slabbekoorn, 2004). Although we did not report results on song amplitude in relation to ambient noise, we found that house finch males modified some song-structure parameters, corroborating previous laboratory and field studies in other species (Sikiba, 2000; Brumm & Todt, 2002; Lohr, Wright & Dooling, 2003; Leonard & Horn, 2005).
House finch males probably raised the low frequency of their songs to minimize noise masking. This type of response has also been found in great tits (Parus major) in the city of Leiden, the Netherlands (Slabbekoorn & Peet, 2003). Narrower bandwidths would also decrease sound masking (Dubois & Martens, 1984; Rheindt, 2003), however, house finch males did not modify song-frequency range. A novel finding of our study was that the number of notes per song decreased rather than increased (see Lengagne, Aubin, Lauga & Jouventin, 1999) with ambient noise. This finding was rather surprising because (a) songs with more notes would increase signal detection, and (b) female house finches prefer males with long songs (Nolan & Hill, 2004). Reducing song length would be expected to have a negative effect on male mating success. One interpretation is that there could be a trade-off between song amplitude and number of notes per song to optimize energy expenditure during the breeding season. Energy could then be allocated to produce louder songs or longer songs, depending upon ambient noise levels. This explanation assumes that singing entails significant energy costs (Oberweger & Goller, 2001), but recent evidence shows that those costs may be minimal (Ward, Speakman & Slater, 2003). Alternatively, we may have found males of low quality with short songs in low-quality (e.g., noisy) areas. However, this explanation is limited by the fact that we were not able to capture males and control for body condition effects and that our amplitude estimates were constrained by some confounding factors (see Methods).
We conclude that house finch males select parks and perches with characteristics that will increase their mating success in urban areas, and change their singing behavior to minimize acoustic constraints due to high noise levels. These behavioral changes are examples of some of the flexible mechanisms this species uses to adapt to urban environments.
Acknowledgments
We deeply thank Laura Apodaca, Kim Xa, Yecenia Gomez, Joey Calmer, Danny Rodriguez, Anna Valcarcel, Angelika Moskova, William Samson, Shelya Jones, and Georgina De La Hoya for their help and effort in gathering data, Lisa Manriquez for coordinating the field crews, Alan Miller for his continuous encouragement to involve undergraduates in ornithological field research, Charlie Collins and Dan Blumstein for fruitful discussions about the project, and two anonymous referees for constructive comments. Esteban Fernández-Juricic was supported by the College of Natural Sciences and Mathematics, California State University, Long Beach (Behavior and Conservation lab website: http://www.csulb.edu/web/labs/bcl/).