Nesting Success and Life-History Attributes of Bird Communities Along an Urbanization Gradient
by Joseph A. Reale¹ and Robert B. Blair²
¹City of Boulder Open Space and Mountain Parks, P.O. Box 791, Boulder, CO 80306
²Department of Fisheries, Wildlife, and Conservation Biology, 200 Hodson Hall, University of Minnesota, St. Paul, MN 55108 (address at time of research: Department of Zoology, Miami University, Oxford, OH 45056)
Abstract
The increase in urbanization in North America has raised concerns regarding impacts on avian populations. In this study, we measured the nesting success of American robins and northern cardinals and analyzed the changes in bird community along an urbanization gradient in southwestern Ohio. We found that nesting failure was not significantly correlated with the gradient, but that it was correlated to nest height, which decreased significantly from the most natural to the most urban sites. We also found that nesting failure was not predicted by the density of adult birds. At the community level, the number of species that use a multiple-brood breeding strategy increased with urbanization. Furthermore, birds identified as high-nesting species reached their highest proportion at the most natural sites and decreased in number with urbanization. In contrast, low-nesting species exhibited the reverse trend. These findings suggest that nesting success—determined by nest site availability and the ability to produce multiple broods—may drive the distribution of avian species along an urbanization gradient, and that nesting site is a critical resource that regulates the distribution of birds in urban environments.
Key words: American robin; community; life histories; nesting success; northern cardinal; southwestern Ohio; urbanization
Introduction
The growth of urban centers in the United States has profound effects on natural ecosystems. Increases in urban populations result in the wholesale conversion of agricultural and forest tracts into urban and suburban environments. The result of this change in land use is a mosaic of land types ranging from entirely built-up urban centers to natural or seminatural areas (McDonnell, Pickett & Pouyat, 1993). Land use for human purposes alters both the structure and function of ecosystems and is the leading cause of biological diversity loss worldwide (Vitousek, Mooney, Lubechenco & Melillo, 1997).
As development reaches into rural areas, many forests, if not fragmented or obliterated outright, are enveloped by human settlement (Friesen, Eagles & Mackay, 1995). This has imposed great stress on avian populations, with many songbird species experiencing declines in some portion of their range (Wilcove & Terborgh, 1984; Askins, Philbrick & Sugeno, 1990; Sauer, Hines & Fallon, 2005). As breeding habitat becomes more fragmented, nest predation increases (Gates & Gysel, 1978; King, Griffin & DeGraaf, 1996; Bayne & Hobson, 1997), brood parasitism increases (Brittingham & Temple, 1983), interspecific competition for resources is more pronounced (Cawthorne & Merchant, 1980; Ambuel & Temple, 1983), and pairing success decreases (Gibbs & Faaborg, 1990; Villard, Martin & Drummond, 1993).
The effects of urbanization on bird communities are well documented (Hoover, Brittingham & Goodrich, 1995; Friesen et al., 1995; Blair, 1996; Morse & Robinson, 1998; Porneluzi & Faaborg, 1999). These studies show that total and native species richness decline at high levels of development. Individual species, however, display differing responses to urbanization. Some birds reach peak densities in urban or suburban settings, while others reach peak densities at natural sites (Mills, Dunning & Bates, 1989; Blair, 1996; Clergeau, Savard, Mennechez & Falardeau, 1998; Gering & Blair 1999).
The cumulative response of individual species to urbanization also results in changes at the level of the bird assemblage. Blair (2001) examined the distribution and abundance of birds along an urban gradient in southwestern Ohio. This study included a spectrum of habitat types created by urbanization, ranging from a pristine nature reserve to a highly developed urban center. Individual species displayed patterns of abundance along the gradient that reflect their level of tolerance for human impact. For example, European starlings (Sturnus vulgaris) were labeled "urban exploiters" based on their higher abundance at the urban end of the gradient. On the opposite end, ovenbirds (Seiurus aurocapilla) were labeled "urban avoiders" based on their high abundance at the natural end of the gradient and their complete absence from the urban end.
The urban bird community is most strongly influenced by vegetation, with the volume of native vegetation being most closely correlated with native bird density and species richness (Mills, Dunning & Bates, 1991). The urban environment favors species that can utilize small, discontinuous patches of vegetation (Beissinger & Osborne, 1982), and densities of urban exploiters are strongly correlated with lawn area and the volume of exotic vegetation (Mills, Dunning & Bates, 1989). The relationship between habitat variables such as vegetation density and species diversity has traditionally been explained in terms of food abundance and foraging niche space (MacArthur, 1961; MacArthur, MacArthur & Preer, 1962; MacArthur, Recher & Cody, 1966; Martin & Karr, 1986). However, Martin (1988b) hypothesized that species distribution may also be influenced by the availability of suitable nesting sites.
Nest predation is the most common cause of nesting failure among open-cup nesting passerines (Ricklefs, 1969; Martin, 1988a). As a result, predation pressure may be an important factor in regulating densities and distributions of birds (Emlen, 1974). The influence of nest predation at the level of assemblage has been largely unstudied (Martin, 1988c). Because the intensity of nest predation varies with attributes of the nesting substrate and nest height (Ricklefs, 1969; Martin & Roper, 1988; Martin & Li, 1992; Martin, 1993a), the effects of vegetation on species distributions may be due in part to the availability of suitable nesting sites (Martin 1988c).
Many researchers have studied the effects of grazing, clear cutting, and other types of habitat alteration on nest predation (for example, see Wilcove, 1985; Hoover et al., 1995; Bayne & Hobson, 1997; Ammon & Stacey, 1997; Zanette & Jenkins, 2000). Recently, ecologists have started investigating these effects in urban settings. Any changes in the assemblage of predators coinciding with increased urbanization (Tomialojc, 1970; Churcher & Lawton, 1987) would be expected to change predation pressure, which in turn may affect overall community structure.
The most common way of assessing community-level predation pressure in birds is by using artificial nests. To measure changes in predation pressure associated with urbanization, Gering and Blair (1999) placed handmade nests containing Japanese quail eggs (Coturnix coturnix) in six sites representing a gradient of urbanization. They found that the overall frequency of nest predation decreased with increasing levels of urbanization. This trend was also supported by a study on predation of actual robin nests (Morneau, Lepine, Decarie & Desranges, 1995). From these studies, they proposed the "predatory relaxation" hypothesis, in which urban environments serve as safe zones with reduced predation pressure because of fewer predators.
Alternatively, Sasvari, Csorgo, and Hahn (1995) measured the predation rate on artificial nests in an urban park and contrasted it with the predation rate in a mixed oak-beech forest. They concluded that reduced species richness of birds in urban environments was a result of increased predation pressure. Their results were supported by Jokimaki and Huhta (2000), who also measured predation rates in a series of parks with different levels of surrounding urbanization. However, based on beak impressions left on plasticine eggs, most of the predation in the town center was attributed to avian nest predators. Willebrand and Marcstrom (1988) demonstrated that birds are the primary predators on artificial nests and that mammals are more often found to prey on real nests. As a result, artificial nests do not always reflect true differences in nest predation among different nest sites and heights (Martin, 1987).
Clear differences in predator response to artificial nests and a lack of correlation between artificial-nest and real-nest predation probabilities are shown in numerous studies (see Major & Kendal, 1996, for a review, and King, DeGraaf, Griffin & Maier, 1999). Furthermore, artificial nests only model predation on the incubation stage of nesting. But nesting failure can be attributed to multiple causes other than predation in the incubation stage, including predation of hatchlings, abandonment due to adult mortality or lack of resources, and destruction of nests through natural catastrophes such as wind or rain. Brood parasites, such as the brown-headed cowbird (Molothrus ater), can also negatively affect the outcome of nesting attempts, and their impact may vary greatly with habitat attributes (Hoover, Brittingham & Goodrich, 1995).
As a result, conclusions about community-level effects of nest failure based on artificial nests may be spurious. To assess the effects of urbanization on nesting failure and related impacts on community organization, data on real nests are needed. The goal of this study was to measure nesting success of real nests and to assess the relationship between nesting outcome and the avian assemblage organization along an urban gradient in southwestern Ohio. To do this, we measured nesting success of real nests and analyzed the composition of bird community along the urban gradient.
Specifically, we addressed the following four questions:
- Does increased urbanization affect the nesting success of urban birds?
- Do nest-site characteristics influence nesting success, and if so, do these characteristics vary across a gradient in urbanization?
- Does the reproductive strategy of birds change with increasing levels of urbanization?
- Is the change in the bird community associated with urbanization correlated with a change in nesting guilds?
Methods
Study Sites
We used an urban gradient previously established by Gering and Blair (1999). It was composed of six 16-hectare sites representing different levels of urbanization. Four of the sites were located within Oxford, Ohio, a small city (14.7 square kilometers) with a population of 21,943 (U.S. Census Bureau, 2000). The other sites—a golf course and a nature preserve—were located seven kilometers north of Oxford. The sites of the urban gradient, listed in order of increasing urbanization, were as follows:
- Nature preserve site—Hueston Woods Nature Preserve
This site was located in a 67-hectare woodlot (Hueston Woods) composed of mature beech-maple forest, with the exceptions of a small parking area, narrow service road, and moderate-use foot trails. The area is encompassed by a state park (975 hectares), which consists of young and mature deciduous forest, old fields, infrequent stands of conifers, and a 250-hectare lake. - Open-space site—Peffer Memorial Park
This site was located in an 80-hectare park that is managed by Miami University, Oxford, as a multiple-use trail system for local residents. The university purchased the park as two separate parcels, a pastureland and an agricultural field, in 1955 and 1966, respectively. The current vegetation is predominantly secondary growth of low stature. The park can be considered a "wildland" because buildings and pavement are absent; however, it has a history of substantial human manipulation. - Golf course site—Hueston Woods Golf Course
This site was located in a 102-hectare golf course seven kilometers from Oxford. The course was established in 1980. Its rough consists primarily of native trees (e.g., maple, beech) and grasses. Less than 2% of its area is covered with buildings and pavement. - Residential district site—Oxford
This 16-hectare site covered approximately ten city blocks within a much larger residential area and mainly consisted of single-family houses built between 1960 and 1974. The vegetation is largely dense lawns, gardens, shade trees, and both native and ornamental flora (Beissinger & Osborne, 1982). The site is suburban, with approximately 32% of its area covered with buildings and pavement. - Apartment complex site—Oxford
This 16-hectare site consisted of a series of multilevel condominium-style apartment complexes with interspersed parking lots, isolated trees, and small landscaped areas. These complexes were developed between 1960 and 1980 and are surrounded by a residential area on three sides and a commercial area on the fourth side. The site is marginally urban, with approximately 61% of its area covered with buildings and pavement. - Business district site—Oxford
This 16-hectare site covered an expanse of four city blocks by two city blocks originally constructed in the mid-1800s, and it is surrounded by an older residential area. It is a distinctly urban site, with approximately 81 percent of the area covered in buildings and pavement. The site is composed primarily of low-rise office buildings, parking lots, ornamental shrubs, and landscape trees.
We applied principal components analysis (PCA) using PC-ORD software (Version 4, 1999) to assign each site a value denoting its level of urbanization. Landscape-level estimates of the percent of cover of trees and shrubs, lawn, buildings, and pavement for each of the sites had been previously obtained (Gering & Blair, 1999). A combination of ortho-digital maps and aerial photographs were used to quantify surface cover. The land cover for each of the sites was broken down into the percent of surface area covered by buildings, pavement, lawn or grassland, and trees or shrubs. The estimates of percent cover were analyzed with PCA, and the scores for each site from the first axis of the analysis were used to assign site values as follows: business district (2.48); apartment complex (1.75); residential district (0.30); golf course (–0.84); and open space (–1.70). We used this approach to create a continuous measure of urbanization of the sites so that we would not be limited to statistical analyses based on rank order alone.
Study Species
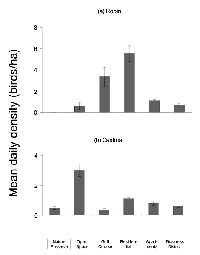
Using abundance data based on Gering and Blair (1999) and Blair (2001), we identified the American robin (Turdus migratorius) and northern cardinal (Cardinalis cardinalis) as two species that were present at most sites along the gradient. These species displayed differing patterns of abundance, with robins being more urban tolerant (appearing at higher densities at the urban end of the gradient) and cardinals showing a preference for the natural end of the gradient (Figure 1).
Nest Location and Fate
We located nests during May and June 2000 by thoroughly searching each 16-hectare site and observing behavioral cues of parental birds, including nest building and vocalization. We marked nest sites by placing flagging within 15 meters of the nests and recording their position with GPS. After locating these nests, we used a convex mirror mounted on a ten-meter expandable pole (Parker, 1972) to determine the contents and to minimize contact with nests.
We discovered all nests during the building, egg laying, or early incubation stages. We monitored nests every three to four days during incubation to determine hatch dates and, after hatching, to determine fledging success. For each nest, we recorded the number of eggs laid, hatched, and fledged as well as any predation events or other instances of egg loss.
Nest Characteristics
After each nesting attempt was completed, we measured habitat characteristics at and around the nest. We recorded the height of the nest and its depth into the vegetation. We used the diameter in centimeters at breast height (DBH) of the nesting vegetation to classify the nest location as a tree (DBH > 10 cm), sapling (10 cm > DBH > 5 cm), or shrub (DBH < 5 cm). We measured the distance from the nest to the nearest tree (DBH > 10 cm) and the nearest edge (defined as an area of open canopy).
Community classification
Gering and Blair (1999) had previously recorded the species present and relative abundance of birds at each site. Using these data, we classified each species according to:
- breeding strategy (single, double, or multiple broods per season)
- nesting height in meters (low: 0 to 3; mid-height: 3 to 6; and high: > 6)
- nest location (tree, shrub/ground, or other—cliffs, buildings, etc.)
- nest type (open or cavity)
Statistical Analyses
Nesting success of robins and cardinals. We analyzed each species separately for the dependency of nest fate (unsuccessful = 0, successful = 1) on site using a logistic regression model (Collett, 1991). The model was specified as follows: Logit (p) = β0 + β1(site) + β2(species) + β3(site) × (species), where p is the proportion of nests that failed (0 < p < 1), with Logit (p) = ln (p/p + 1). "Site" is a continuous variable for site based on the PCA value. "Species" is an indicator variable that discriminates between species (i.e., species = 0, for cardinals, and species = 1, for robins).
We used a separate logistic regression model to determine if species density predicted nest fate. The model was specified as follows: Logit (p) = β0 + β1(density), where p is the proportion of nests that failed (0 < p < 1), with Logit (p) = ln (p/p + 1). Density is the density of the species being tested.
We further analyzed each species for dependency of nest fate on nest characteristics. The full model was specified as Logit (p) = β0 + β1 (H) + β2 (D) + β3 (E) + β4 (T) + β5 (V), where p is the proportion of nests in the stage being tested that failed (0 < p < 1), with Logit (p) = ln (p/p + 1). The letters H, D, E, and T are continuous variables for nest height, depth of nest in vegetation, edge distance, and distance to the nearest tree, respectively. V is a categorical variable representing vegetation type, (V = 1 for shrubs, V = 2 for saplings, and V = 3 for trees).
Starting with the full model, we used Wald's statistic (W) to identify significant variables (p < 0.05). We eliminated variables stepwise from the model until only significant variables remained. Using a one-way ANOVA, we tested variables that were significant in predicting nesting success for differences in their mean values across sites. We also used linear regression models to determine whether nest characteristics that were significant predictors of nesting success were related to the degree of urbanization.
Nesting guilds. We analyzed the community classifications using a chi-square (Χ2) test for independence (Samuels & Whitmer, 1999). The null hypothesis for all comparisons (H0) was that the proportion of individuals with a given nesting characteristic was equal to the proportion of individuals in the entire population with that trait (i.e., assemblages were random). We calculated expected values by multiplying the number of species present at a given site by the relative proportion of all species that possessed the trait being tested. For example, 30 out of 42 total species (71.5%) were open-nesting species. The nature preserve had 17 species present, so we expected that 71.5%, or 12.14 species, would be open nesting. We used community characteristics that varied significantly across sites in a linear regression model to determine if they were significantly correlated to the degree of urbanization.
Results
Nesting Success
We discovered nests in five of the six sites. At the nature preserve, robins were absent and cardinals were present in very low numbers and restricted to a small portion of the preserve. Therefore, we did not include data on nesting success from the nature preserve.
We discovered 85 nests in the five remaining sites: 51 robin nests and 34 cardinal nests. Across all sites, 65% of robin nests and 71% of cardinal nests were successful (Table 1). Density was not a significant predictor of nest fate for robins (DF = 1, W = 0.08, p = 0.67) or cardinals (DF= 1, W = 0.01, p = 0.84). The site was also not a significant predictor of nest fate for robins (DF = 1, W = 0.66, p = 0.42) or cardinals (DF= 1, W = 0.002, p = 0.96).
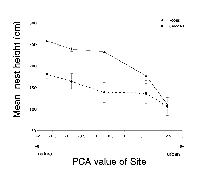
Nest height was the only significant predictor of nest success for both species (robins: DF = 1, W = 14.157, p < 0.01; and cardinals: DF = 1, W = 9.410, p < 0.01). In both species, higher nests were more likely to be successful.
Mean nest height for robins and cardinals differed significantly across sites (robins: DF = 4, F = 14.62, p < 0.001; cardinals: DF = 4, F = 4.94, p = 0.03). The mean nest height between the two species was significantly different (DF = 1, F = 10.06, p = 0.002). For both species, the mean nest height decreased with increasing levels of urbanization (robins: R2 = 0.23, p < 0.001; cardinals: R2 = 0.13, p = 0.03) (Figure 2).
Nesting Guilds
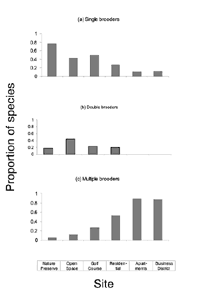
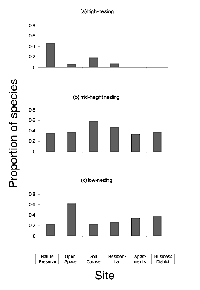
Blair (2001) detected 43 species across all six sites of the urban gradient. We eliminated brown-headed cowbird from the guild analysis because of its unique status as a brood parasite (see Table 2 for species classifications). We classified 29% of the species as high nesting, 33% as mid-height nesting, and 37% as low nesting. Tree-nesting species accounted for 55% (n = 23), and shrub/ground-nesting species made up 31% (n = 13). We classified an additional 14% (n = 6) as having some other nesting location. The population was 55% (n = 23) single-brooding, 24% (n = 10) double-brooding, and 21% (n = 9) multiple-brooding species.
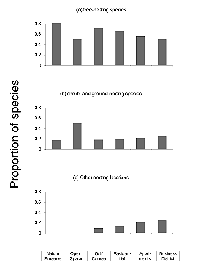
The brooding strategy of species differed significantly across sites (DF = 10, X2 = 61.12, p < 0.001). The proportion of single-brooding species declined dramatically with increased urbanization. Double-brooding species peaked in the open-space area and were entirely absent from the most urban sites. There was a strong trend toward an increasing proportion of species using a multiple-brood strategy with increased urbanization. The number of broods a species attempts in a year was related to the degree of urbanization (R2 = 0.93, p < 0.01) (Figure 3).
The nesting-height guild differed significantly across sites (DF = 10, X2 = 27.73, p < 0.01). The proportion of species using high nest sites peaked at the most natural sites and declined with increasing urbanization, with no high-nesting species present in the two most urban sites. The proportion of mid-height-nesting species reached peak values at the moderately urbanized sites (residential district and golf course) while remaining relatively constant at the extreme ends of the gradient. Low-nesting species exhibited peak proportions at the open-space site and showed a general increase from the golf course to the business district. There was a marginally significant inverse correlation between nest height and the degree of urbanization (R2 = 0.96, p = 0.06) (Figure 4).
Nesting location did not differ significantly across the sites (DF = 10, X2 = 15.64, p = 0.09). Tree-nesting species were evenly distributed across sites. The proportion of shrub- and ground-nesting species was greatest at the open-space site but was otherwise evenly distributed. However, none of the species with unique nesting locations was present at the least urban end of the gradient, and their relative numbers increased with increasing urbanization (Figure 5). The distribution of cavity-nesting and open-nesting species did not differ significantly across sites (DF = 5, X2 = 5.91, p = 0.11).
Discussion
Many factors may act as selective forces in determining the nesting outcome, habitat selection, and community structure of birds. These include the availability of adequate forage (Lack, 1954; Martin, 1987), interspecific competition for resources (Holmes, Sherry & Sturges, 1986; Moulton & Pimm, 1986), and the availability of vegetation and habitat, which provides cover and nesting or feeding substrate (Karr & Roth, 1971; Mills, Dunning & Bates, 1991). In urban ecosystems, the abundance and diversity of vegetation and habitat heterogeneity affect the species richness and diversity of birds (Lancaster & Rees, 1979; Dowd, 1992). Local features are more important than landscape-level features in determining the composition of urban-bird communities (Clergeau et al., 1998).
In our study, the first question we asked was whether increasing levels of urbanization influenced the nesting success of birds. Although nesting success varied widely for robins (43% to 73%) and cardinals (56% to 78%), the variation was not attributable to location along the urban gradient. Furthermore, the relative abundance of each species was not predicted by nesting success. Our findings fail to support the conclusion that nesting success influences the relative density of individual species. This means that individuals within each species are probably unable to select nesting sites based on the suitability of the landscape in terms of nesting success. Further, this result has implications concerning the use of density measurements to indicate habitat quality. These findings support the review by Bock and Jones (2004) that bird densities generally are good predictors of nesting success except in human-dominated environments.
However, in answer to the second question of our investigation, nest height significantly influenced nesting outcome for both species, with higher nests being more likely to succeed. At the same time, mean nest height decreases with urbanization. Consequently, as sites become urbanized, individual birds are forced to choose nesting locations that decrease their chances of success. This may be due to a lack of available nesting sites.
Third, we asked if reproductive strategy changed with urbanization. While neither of our study species—robins and cardinals—showed any difference in nesting success across the gradient, the proportion of species in the entire bird community that rely on a strategy of multiple brooding dramatically increased with urbanization. Both robins and cardinals are suburban-adaptable species that actively defend their nests, so they may not adequately represent the entire avian assemblage. Single-brooding species show a strong preference for the most undisturbed sites, and their numbers decrease with urbanization. This shift in the overall breeding strategy of the community is due, in large part, to a loss of migratory species in more urban sites. Multiple-brooding species may be able to compensate for nesting losses in habitats with low nesting success, while most neotropical migrants are restricted to one or two broods per season because of the energy costs associated with long-distance migration (Whitcomb, Robbins, Lynch, Klimkiewicz & Bystrak, 1981; Terborgh, 1989).
The change in breeding strategy from low productivity (single brood) in undisturbed sites to high productivity (multiple broods) in urban sites may indicate a change in overall nesting-habitat quality. The strategy change may imply that increased urbanization results in a decrease in nesting success. Species that are able to afford the loss of one or more broods per season may be better adapted for urban sites, while those that rely on a single brood each year may be unable to maintain populations in urban areas.
Consistent with the findings of other studies (e.g., Ricklefs, 1969; Skutch, 1985; Martin, 1988b), nest predation was the primary cause of nesting failure for all sites. Because of the potentially enormous influence of predation on avian assemblages, the community-level influences of nest predation have become a primary focus in avian studies. It has been hypothesized that avian community organization may be regulated by the availability of nest sites that minimize the risk of predation (Martin, 1992). The results of this study suggest that nesting success over the entire breeding season rather than of individual nests may drive the distribution of avian species in urbanizing environments.
The variation in the avian assemblage that occurs in conjunction with variations in vegetation has been attributed to differences in foraging strategy (MacArthur, 1961; MacArthur, MacArthur & Preer, 1962; MacArthur, Recher & Cody, 1966; Sabo & Holmes, 1983). However, the availability of suitable nesting sites may be more limiting than food (Rosenberg, Terrill & Rosenberg, 1987). Most birds are highly specialized in their nesting-site location, while foraging preferences are more generalized and exhibit greater interspecific overlap (Martin, 1988a; Martin, 1993b). After comparing residential neighborhoods of Oxford and the nature preserve, Beissinger and Osborne (1982) concluded that the vertical distribution of vegetation could not explain the decrease in avian species diversity associated with urbanization. However, the total volume of vegetation was significantly lower in the residential area, and this may have been a factor limiting food availability, cover, and nest placement. The changes in nesting-height guild across the urban gradient in our study are consistent with the hypothesis that the availability of suitable nesting sites is a selective factor in determining the composition of the avian bird assemblage in urbanizing environments.
At the most urban end of the gradient, low-nesting species completely replace high-nesting species. The transition from undisturbed woodland to a highly modified business district results in a shift in vegetation from tall, native trees and sparse understory vegetation to short, isolated ornamental trees and shrubs (Beissinger & Osborne, 1982; Gering & Blair, 1999). This should favor species that can make use of lower nesting sites. In addition, the introduction of novel nesting locations (i.e., chimneys, dryer vents, rain gutters, and other man-made structures) allows species with unique or flexible nesting preferences such as starlings, house sparrows (Passer domesticus), chimney swifts (Chaetura pelagica), and rock doves (Columba livia) to inhabit highly urban sites.
The results of this study indicate that nesting site is a critical resource that regulates the distribution of birds in an urban environment. The habitat alteration that accompanies urbanization reduces the diversity and availability of nesting sites. This may force individuals to use poor-quality nest sites, and our data suggest that this may alter the species of birds in the community. It may be possible to increase the numbers of native species in urban settings by using more native plants in the landscape and increasing the volume of vegetation that provides optimal nesting sites.
Acknowledgments
We wish to thank Dr. A. John Bailer, Dr. Dennis Claussen, and Dr. Tom Crist for their advice and suggestions; John Minturn, for his tireless fieldwork; Sean Walker for his patient statistical advice; Jon Gering for design and editing support; and Valerie Tierce, Nancy Crosby, and Chris Betrus for editing, varied support, and kindness. This project was financed in part by the Department of Zoology and the Undergraduate Summer Scholars Program at Miami University.