Forest Fragmentation Effects on Ovenbird Populations in the Urban Region of Eastern Massachusetts, USA
by David C. Morimoto1, Michelle A. Frankel2, Marta Hersek3, Fred E. Wasserman3
1 Natural Science and Mathematics Division, Lesley University, Cambridge, MA 02138
2 Audubon Connecticut, 613 Riversville Road, Greenwich, CT 06831
3 Department of Biology, Boston University, 5 Cummington St., Boston, MA 02215
Abstract
We compared pairing and reproductive success of ovenbirds (Seiurus aurocapilla) in three large (120‒312 hectare) and nine small (10‒60 hectare) forest reserves in a suburban landscape over six years and related ovenbird success to patch-scale and landscape-scale features. We applied estimates of ovenbird reproductive success to population viability models and compared results with those of ovenbird studies in other regions and landscapes. Pairing success was high at all sites but not significantly higher in large (98%) vs. small (88%) reserves. The probability of nest survival was significantly higher in large (42%) vs. small (17.5%) reserves, as were reproductive success (61.3% vs. 46.8%) and fledging success (70% vs. 50%). Density was significantly higher and territories were significantly smaller in large reserves. The amount of forested area within 2 kilometers of the forest center was somewhat positively related to the proportion of successful nesting attempts and fledging success (p < 0.10). There was no significant difference in predation or parasitism rates by brown-headed cowbirds (Molothrus ater) between large and small forests, but parasitized nests in small reserves fledged significantly more cowbird nestlings. Source-sink results varied with estimates of adult survivorship and annual productivity, but most models found that large reserves were above the source-sink threshold and small sites were population sinks or very near the source-sink threshold. Suburban landscapes in heavily urbanized regions of the northeastern U.S. can likely support viable populations of ovenbirds with forest cover > 40%, with the maintenance of reserves ≥ 120 hectares, and with the preservation of small woodlots close to larger tracts of forest. The rates of pairing and breeding success in our irregularly patterned suburban landscape were higher than those found in other regions and landscapes, supporting the conclusion that regional and landscape context are important considerations in the conservation and management of ovenbirds, and pointing to the importance of local and regional studies for determining minimum area requirements for ovenbirds and for informing municipal planning and conservation efforts.
Keywords: forest fragmentation, ovenbirds, conservation, minimum-area breeding requirements, source-sink, urban
Introduction:
Forest fragmentation is a major cause of population declines in many species of neotropical migrant birds in North America (Whitcomb et al. 1981; Lynch and Whigham 1984; Askins et al. 1990; Hagan and Johnston 1992; Bayne and Hobson 2001a; Nol et al. 2005; Sauer et al. 2005; Zuckerberg and Porter 2010). Given a world of continuing human development and the need for sound planning and management to conserve biodiversity, it is therefore important to determine minimum-area breeding requirements for birds and to identify landscape and vegetation features that support viable breeding populations (Robbins et al. 1989, Robinson et al. 1995; Donnelly and Marzluff 2004; Reale and Blair 2005). Landscape-scale studies are critical in order to detect fragmentation effects on bird populations, yet they are less numerous than patch-scale or edge-scale studies (Stephens et al. 2003). The type of landscape (e.g., forested/agricultural/exurban, rural/ruderal, suburban, urban) can interact with reserve size, amount of regional forest cover, and nest-site availability to influence reproductive success and patterns of occurrence. The need for landscape scale studies of birds is perhaps most important in urban regions (e.g., Fernández-Juricic and Jokimäki 2001; Crooks et al. 2004; Feldman and Krannitz 2004; Reale and Blair 2005), where expansion of human populations is greatest (Vitousek et al. 1997; Grimm et al. 2008; Forman 2008). Yet, while many studies have explored the effects of fragmentation on birds in urban regions (Marzluff 2001), relatively few have quantified source-sink dynamics for individual species in these urban and suburban landscapes.
Suburban landscapes have "softer" or more complexly contoured forest edges than agricultural landscapes; forests often grade into wooded residential lots and create an irregular patterning of habitats (McIntyre and Barrett 1992; McDonnell, Pickett and Pouyat 1993; Fischer et al. 2004). Irregularly patterned landscapes therefore may be less susceptible to the edge or area effects commonly associated with nest depredation and the decline of some forest species and thus may be able to support more native birds than do relatively isolated forest patches in agricultural or clear-cut forest landscapes (Robinson et al. 1995; Donnelly and Marzluff 2004). These irregularly patterned landscapes are changing, however, with the rapid destruction of forests for residential development such as in the urban regions of the northeastern United States (Steel 1999). Thus, the need to assess the population viability of migrant songbirds in irregularly patterned landscapes within urban regions is important.
Most studies of ovenbirds (Seiurus aurocapilla), a fragmentation-sensitive neotropical migrant wood warbler (Van Horn 1994), have been conducted in agricultural landscapes with relatively low regional forest cover (Gibbs and Faaborg 1990; Villard et al. 1993; Wenny et al. 1993; Van Horn et al. 1995; Bayne and Hobson 2001a; Nol et al. 2005; Perot and Villard 2009), or in forested landscapes undergoing sylviculture (Merrill et al. 1998; Flaspohler et al. 2001; Robinson and Robinson 1999; Mazerolle and Hobson 2002; Vanderwel et al. 2007). Minimum area requirements for the ovenbird have varied in the literature (Robins et al. 1989; Porneluzi and Faaborg 1999) and may not apply across the breeding range of this species.
Based on studies in the midwestern USA (Donovan et al. 1995, Porneluzi and Faaborg 1999), the minimum-area estimate for viable ovenbird populations is 350 hectares. At the municipal scale, it is often impossible for townships in many urban regions to set aside conservation areas large enough to meet these reported minimum-area requirements. It was our goal to determine the viability of ovenbird populations in a 41%-forested suburban landscape within the urbanizing region of eastern Massachusetts, and to identify landscape features associated with patterns of reproductive success. We examined density patterns and the pairing, reproductive, and fledging success of ovenbirds in forested sites ranging from 10 to 312 hectares, and related these values to landscape variables. We applied estimates of ovenbird population parameters in this landscape to population viability models, and we discuss the implications of our findings for land managers, planners, and residents of forested urban regions in the northeastern United States.
Methods
Study Area
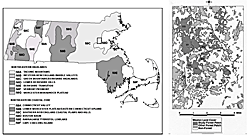
The study was conducted primarily in the suburban town of Weston, with one site each in Wayland and Wellesley, in Middlesex County, Massachusetts, 15 kilometers west of Boston (Figure 1). All sites were located on municipal conservation land (reserves), with some small- scale silviculture occurring. The study area is within the New England Coastal Plains and Hills bioregion in a transition zone with the Boston Basin, a heavily urbanized (80% developed) bioregion (Griffith et al. 1994). The landscape is approximately 41% forested, and wooded areas are bordered primarily by roads and large residential lots, with some small satellite commercial districts and small agricultural fields < 20 hectares; Figure 2).
Most of the sites contain permanent streams or swamps and have moisture gradients with associated plant communities ranging from red maple (Acer rubrum) swamp forest to drier upland oak-hickory forest (Morimoto 1992). The forests are dominated by red oak (Quercus rubra), red maple, and white pine (Pinus strobus). Dominant understory shrubs include blueberry (Vaccinium spp.), black huckleberry (Gaylussaccia baccata), and mapleleaf viburnum (Viburnum acerifolium), while the dominant herbs include Canada mayflower (Maianthemum canadense), and partridgeberry (Mitchella repens), as well as club mosses (Lycopodium spp.).
Ovenbird Monitoring
We monitored 20 forested reserves for ovenbird pairing and reproductive success in each of six years (1994‒1997, 1999, 2000) and included in the analyses any site in any year that was found to have at least one singing male ovenbird occurring on a territory well into the breeding season (mid-June), for a total of twelve sites (Table 1). No reserve < 10 hectares contained singing male ovenbirds into the breeding season (mid-May). The irregularly patterned nature of this landscape made it difficult to delineate distinct patches or fragments per se, but each of the sites was separated from nearby forest by at least one road and by residential or commercial development or by small open fields (Figures 1 and 2).
The 12 sites included in the final analyses ranged in size from 10 to 312 hectares and included 3 large reserves (> 120 hectares) and 9 small reserves (< 60 hectares; Table 1), with size classes determined by natural breaks in the frequency distribution of fragment sizes. While this size categorization may only be appropriate for more heavily forested suburban regions toward the core of the breeding range of ovenbirds, it is similar to that used by Mattsson and Niemi (2006). Donnelly and Marzluff (2004) categorized reserves of 34.7 ± 6.0 hectares as being medium in size, but none of the fragments in their small size class (2.1 ± 0.6 hectares) supported breeding ovenbirds in our study area. The three large sites in our study were the largest forested conservation tracts in the study area. Due to resource constraints and in some years small-scale sylviculture at the time of the study, reserves were not monitored in every year (Table 1). In addition, ovenbirds did not consistently breed in all of the small reserves annually.
Pairing and reproductive success of ovenbirds were measured from early May to late July, 1994‒1997 and 1999‒2000. We studied all ovenbirds in the small fragments and ovenbirds within 24-hectare plots in the three large fragments. Each captured bird received a numbered U.S. Fish and Wildlife band and a unique combination of colored bands for individual identification. Pairing success was determined by monitoring males for 90-minute periods 3 times per week from their time of arrival until late June during the 1994‒1996 field seasons. In 1999 and 2000, pairing success was determined by observing males for 30-minute periods 5 times per week. A male was considered paired if he was seen associating with a female on his territory on at least two occasions. Pairing status was usually confirmed by observing a subsequent nesting attempt. These strict criteria for determining pairing status were necessary since males were often seen associating with neighboring females, even in their own territories. For the analyses of pairing success, we included only males that remained on their territories through the third week in June, since unpaired males were often seen singing and defending territories in the small fragments for the first few weeks of the breeding season and subsequently disappeared. Since these males may have been transients or unpaired floaters, we did not include them in our analyses.
The reproductive status of males was determined by visiting each territory at least twice per week for 90-minute periods until the end of July in 1994‒1996, and 5 times per week for 30-minute periods in 1999 and 2000. During each observation period we searched for nests as well as for signs that males were caring for young. Reproductive success was measured in three ways:
- Mayfield method (Mayfield 1975): Nests were checked three times per week and then every day just prior to fledging in order to determine daily nest mortality rates (total # failed nests/total # days the nests were observed). We also calculated the probability that a nest would succeed as daily survival rate (1 ‒ daily mortality rate) raised to the power of the number of days in the nesting cycle (for ovenbirds, 25 days as per Hann 1937).
- Nest success: the proportion of successful nesting attempts/total # nesting attempts observed. This estimate accounts for the occurrences of multiple nesting attempts per breeding male (Podolsky et al. 2007).
- Fledging success: the proportion of males that have successfully fledged at least one young, regardless of the number of attempts. Unpaired males are included in this measure.
We calculated the average number of nestlings per successful nest in both large and small sites by counting the number of ovenbird nestlings at day five or six of the nestling period, and we included in the calculation only those nests in which at least one ovenbird nestling had fledged. We also recorded the number of brown-headed cowbird eggs and nestlings in each nest and compared the proportion of parasitized nests in large versus small fragments.
Annual Productivity
We utilized three different methods for calculating annual productivity (the number of juvenile females per adult female per year) to allow comparisons with other studies (Donovan et al. 1995; Porneluzi and Faaborg 1999; Podolsky et al. 2007):
- Mayfield estimate: This method utilizes the Mayfield estimate of nesting success and the mean number of female young produced per nest to calculate annual productivity (Donovan et al. 1995). This method assumes that ovenbirds can raise only one brood per year, and that females may re-nest once if their first nesting attempts failed.
- Season-long fecundity: This method estimates nesting success based on the season-long fecundity of individual birds, rather than based on Mayfield data (Porneluzi and Faaborg 1999).
- Observer-effects method: The Mayfield estimate of nesting success does not take into account the effects observers may have on nesting success (Bart and Robson 1982; Rotella et al. 2000). We therefore used a maximum-likelihood estimate of daily survival rate and observer effects, where observer effects are calculated by recording the number of nest failures and successes after different intervals of nest visits (Rotella et al. 2000). We then used the observer-effects estimate of nest success to calculate annual productivity and compared the results to those calculated using the Mayfield and season-long fecundity estimates.
Source-Sink Dynamics
In order to assess whether the large and small reserves were population sources or sinks, we applied the following equation to all large reserves combined and all small reserves combined: λ = PA + PJβ, where λ is the finite rate of increase of a population, PA is the probability of adult female survival, PJ is the probability of juvenile female survival from fledging to the following breeding season, and β is the proportion of juvenile females per adult female per breeding season (Pulliam 1988). If recruitment of young exceeds adult mortality, then the population is a source (λ > 1); if recruitment of young is less than adult mortality, then the population is a sink (λ < 1; Pulliam 1988; see References for calculations of female survival, juvenile survival, and the proportion of juvenile females per adult female)
Landscape Measures
All landscape measures were calculated from 1:25,000 scale MassGIS coverages (Table 2). ArcGIS was used to calculate forest area, regional forest area (amount of forested area within a 2-kilometer radius of the center of a forest), and donut area (proportion of the area within 1 kilometer of the forest boundary that is forested; Federowicz 1999). A radius of 1 kilometer was used for donut area to avoid overlapping areas among sites. Fragstats version 2.0 (McGarigal and Marks 1994) was used to calculate four additional metrics for each site: core area (area of forest that is > 50 meters from the forest perimeter), fractal index (fractal dimension of the forest perimeter, a patch-scale metric), nearest forest distance (the distance, edge to edge, to the nearest forest), and proximity index (for all forests within 1 kilometer of a focal forest, the sum of forest area divided by the distance to the focal forest squared; a forest in close proximity to several large forests will have a large proximity index).
Data Analysis
We compared density, pairing success, nest (reproductive) success, and fledging success in large vs. small reserves using Mann Whiney U-tests. Nest survival rates calculated by the Mayfield method were compared in large vs. small reserves using the program Contrast (Sauer and Williams 1989), which utilizes a chi-square test. We compared the average number of ovenbird nestlings per successful nest and the average number of cowbirds per nest in large vs. small reserves using t-tests. To test the relationship between the landscape variables and ovenbird pairing, nesting and fledging success, we calculated Spearman rank correlation coefficients between the landscape variables and the proportion of paired and successful males at each reserve. We included only sites for which we had data for five or more males in these correlations.
Results
Breeding Success in Large vs. Small Reserves
The density of breeding male ovenbirds was significantly higher in the three large reserves than in the three small reserves (Xlarge = 0.459 ± 0.146; Xsmall = 0.079 ± 0.041; n1 = 10; n2 = 27; U = 270; z = 4.62; p < 0.001; Figure 3). What minimal annual variation in density occurred did not affect these patterns since the small reserves had so many fewer ovenbirds. Although the Spearman rank correlation of average density with annual productivity for the seven sites having data for at least five males (combined across years) was not significant (rs = 0.616; df = 5; p = 0.13), a scatterplot reveals that the three large reserves where density was highest had three of the four highest annual productivity levels (Figure 4). Similar patterns resulted with scatterplots of average density by reproductive success and average density by fledging success for these seven sites. Average territory size was significantly smaller in large reserves (χ2 = 1.43 hectares ± 0.22, n = 17 in large forests; χ2 = 2.26 hectares ± 0.25, n = 13 in small forests; t = 8.02, p < 0.009).
Click images to enlarge
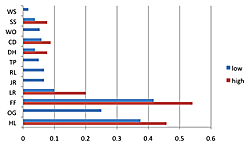
Figure 3. Figure 3. Ranges in the density of breeding male ovenbirds. Some sites (WO, RL, JR, OG) did not vary or had breeding male(s) in only one year (WS, TP). The density of breeding male ovenbirds was significantly higher in the three large reserves than in the three small reserves (n1 = 10, n2 = 27, U = 270, p < 0.001).
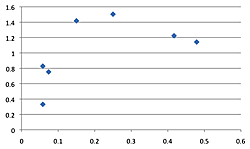
Figure 4. Scatterplot of average male density (x-axis) by annual productivity (y-axis) for the seven reserves having data for at least five males. The three large reserves are the three right-most points (FF, HL, OG, LR, SS, CD, DH, right to left). Similar patterns resulted with scatterplots of average density by reproductive success and average density by fledging success for these seven sites.
Ovenbird pairing success (proportion of paired males) was very high at all sites and not significantly different between large and small reserves, but both reproductive and fledging success (the proportion of successful nests and the proportion of nests that fledged at least one young, respectively) were significantly higher in large reserves than in small reserves (Table 3).
In total, 87 nests were included in the Mayfield estimates of nest survival rates, with 770 nest observation days in the large sites and 312.5 nest observation days in the small sites. The daily survival rate for nests was significantly higher in large (0.97) than in small (0.93) reserves (χ2 = 28.5, df = 1, p < 0.0001), and the probability that a nest survived from egg-laying through fledging was 42% in large sites and 17.5% in small sites. The average clutch size did not differ between large (3.34, n = 35) and small (3.2, n = 10; t = 0.318, p = 0.75) reserves.
Annual Productivity
Because there were no differences in clutch size between large and small reserves, we used the average clutch size for large and small reserves combined (3.31) in our calculations of annual productivity (see References for details of the specific calculations). Annual productivity estimates were higher for large than for small reserves (Table 4). A positive observer effect was detected in large sites (h = 1.017) and a negative observer effect was detected in small sites (h = 0.962). The observer-effects estimates of annual productivity were lower than those of the Mayfield estimates, with season-long fecundity estimates being intermediate (Table 4).
Source-Sink Dynamics
Estimates of the finite rate of increase in ovenbird populations (λ) were higher for large reserves than for small reserves, regardless of the estimation method (Table 5). The finite rate of increase was > 1.0 for large and small reserves using the upper-level estimate of annual productivity (0.854), and ≤ 1.0 when using the intermediate estimate of annual productivity (0.623). As with annual productivity, the observer-effects method reduced the difference in estimates of λ between large and small sites (Table 5).
Nest Predation and Cowbird Parasitism
Nest failure was primarily due to predation; out of 62 failed nests for which the cause of failure could be determined, 48 (77%) failed due to predation, nine (14.5%) failed due to abandonment, two (3.2%) failed due to cowbird parasitism, two (3.2%) were found with nestlings dead in the nest due to unknown causes, and one (1.6%) failed due to flooding of the nest. No differences were found in either the overall predation rates (% total nests depredated) between large (26.5%) and small (21.0%) reserves (χ2 = 0.532, df = 1, p = 0.466) or the predation rates (proportion of failed nests depredated) between large (81.6%) and small (76.2 %) reserves (χ2 = 0.242, df = 1, p = 0.623).
Out of 77 nests for which cowbird parasitism status could be determined, 22 (29%) were found to contain at least one cowbird egg. The average number of cowbird eggs per parasitized nest was 1.6±0.6, and the average number of cowbirds fledged from parasitized nests was 1.3±0.48. Parasitized nests fledged fewer ovenbird nestlings (2.3 ± 0.9, n = 13) than unparasitized nests (3.6 ± 1.3, n = 33; t = 3.323, df = 44, p = 0.002). Of the parasitized nests that successfully fledged young (either cowbird or ovenbird), nests in small sites fledged a significantly higher number of cowbird nestlings (1.75 ± 0.5, n = 4) than nests in large sites (1.11 ± 0.3, n = 9; t = 2.754, df = 11, p = 0.019). The number of total young (cowbirds and ovenbirds) fledged per successful nest was not significantly different between large (3.53 ± 1.2, n = 36) and small (3.9 ± 1.4, n = 10) fragments (t = 0.838, df = 44, p = 0.4). There was no significant difference between the proportion of parasitized nests in large (25.9%, n = 58) and small (36.8%, n = 19) fragments (p = 0.39; Hersek et al. 2002).
Landscape Effects
Only the seven sites with data on five or more males were used in the Spearman rank correlation analyses relating measures of reproductive success to landscape variables (Table 2). Using p = 0.10 as a cutoff value, which is justified given the small sample sizes and pooling across years, regional forest area was positively related to the proportion of successful nesting attempts (rs = 0.775, p < 0.1) and to the proportion of males that fledged at least one young (rs = 0.714, p < 0.1). While area was positively correlated with the proportion of paired males (rs = 0.556), the relationship was not significant (p > 1.0).
Discussion
The relatively high pairing and reproductive success in our study sites (Table 3), when compared to such rates in other studies, supports the conclusions that regional landscape context is important in influencing population viability and that forested suburban landscapes are capable of supporting viable populations of ovenbirds. Pairing success in our study sites was generally higher than that found in similar-sized fragments and as high as or higher than that found in contiguous forests in other studies of ovenbirds conducted in other landscapes (Wander 1985; Villard et al. 1993; Porneluzi et al. 1993; Hagan et al. 1996; Gibbs and Faaborg 1990; Donovan et al. 1995; Ortega and Capen 1999; Van Horn et al. 1995; Sabine et al. 1996; King et al. 1996; Porneluzi and Faaborg 1999; Bayne and Hobson 2001a). Many of the forest fragments in these other studies were > 150 hectares, and yet the pairing success in many of them was lower than what we found even in our small (< 60 hectares) suburban reserves.
Van Horn et al. (1995) compared forest fragments in a highly fragmented, agricultural landscape in Missouri with 23% forest cover. Their small fragments (150‒350 hectares) had a pairing success rate of 15%, compared to 65% in a large (900-hectare) fragment that was contiguous with forest tracts on two sides. In contrast, and similar to what we found in our study, Sabine et al. (1996) found no difference in pairing success rate between their small (> 40 hectares) forest fragments (82% pairing success) and a large contiguous forest (84% pairing success) in a highly forested landscape in southern New Brunswick, Canada. Porneluzi et al. suggest that the deleterious effects of forest fragmentation may occur in much smaller fragments in highly forested landscapes than in forest fragments surrounded by non-forested land (Porneluzi et al. 1993; see also Donnelly and Marzluff 2004; Villard et al. 2007). Bayne and Hobson (2001a) found overall high rates of pairing and a significant difference in pairing success between fragments created by agriculture or forestry and contiguous forests, with rates similar to those found in our study. They attributed the differences between pairing success in fragments and in contiguous forest to differences in floater populations (more floaters in contiguous forest) and differences in habitat quality affecting males' abilities to attract mates (lower quality habitat in fragments).
The values we found for the proportion of breeding pairs that successfully raised young in our large and small forest reserves (74% and 58%, respectively) were similar to those found by Porneluzi and Faaborg (1999), who compared breeding success in a contiguous forest (almost 2 million hectares) in the Missouri Ozarks to forest fragments > 2000 hectares in a highly fragmented landscape (70% and 50% breeding success, respectively). However, our values were lower than the values (82‒92%) reported by Stodola et al. (2010) in contiguous forest in North Carolina.
The relatively high rates of pairing, reproductive, and fledging success that we found in our study sites are likely due to the relatively high forest cover (41%) and high connectivity between reserves in our study area and to the fact that most fragments were surrounded by large residential lots with substantial canopy cover. In this type of landscape, forests may be (1) less permeable to edge predators than isolated forest fragments in agricultural landscapes (Robinson et al. 1995; Chalfoun et al. 2002; Donnelly and Marzluff 2004; see also Villard et al. 2007) and (2) overall permeable to ovenbirds (Gobeil and Villard 2002).
The importance of regional forest coverage in mitigating fragmentation effects is further supported by our analysis of the relationship between landscape variables and measures of breeding success. Despite small sample sizes (n = 7 sites with data for 5 or more males across years), regional forest area, a landscape-scale metric, was correlated, albeit at the p < 0.10 level, with measures of success (proportion of successful nests, proportion of males successfully fledging at least one young), whereas patch-scale metrics such as forest area or fractal dimension had no significant correlations. Mattsson and Niemi (2006) also found significant relationships between landscape variables (amount of core forest) and nest predation rates in ovenbirds. The results also lend some support to the conclusion that landscape scale metrics are more likely to detect fragmentation effects than are patch-scale or edge-scale metrics (Stephens et al. 2003).
The different methods of estimating annual productivity yielded varying results, with the Mayfield method yielding lower estimates than the season-long fecundity and observer-effects methods (Table 4). The Mayfield estimate of nest success likely underestimates annual productivity. First, it assumes that ovenbirds with failed nests only re-nest once. Podolsky et al. (2007) emphasize the importance of using models that account for re-nesting when estimating annual productivity and the finite rate of increase. Indeed, we observed several instances of re-nesting through the course of the study (17.4% re-nesting in large reserves, 20% in small sites), with one instance (in a small reserve) in which ovenbirds had three nesting attempts in a season. Second, the Mayfield estimate of productivity may be an underestimate since it does not take into account the negative effects an observer may have on the survival probability of a nest. The observer-effects model did find a negative observer effect on nests in small sites, and when this effect was taken into account, the resulting annual productivity estimates for large and small reserves were closer in value. The slight positive observer effect that was found in the large reserves could be due to avoidance effects of observers on predators.
What is ultimately important for understanding fragmentation effects on ovenbirds is whether or not the populations can sustain themselves over time. Our source-sink analyses suggest that, depending on the method used, the large reserves we studied are either sources (λ > 1) or at or near the source-sink threshold (λ = 1), and the small reserves are just below the source-sink threshold (Table 5). Without more precise and direct estimates of adult female and juvenile survival rates for our study area, it is difficult to know which of the above models best applies to our system; estimates of λ are highly sensitive to estimates of survival rates (Flaspohler et al. 2001; Podolsky et al. 2007). However, analyses of return rates data from our largest study site for 1997‒2000 yield an average annual male return rate of 0.853 (Frankel, 2001), which is very close to the survivorship estimate of 0.845 found by Roberts (1971) in a study also performed in Massachusetts. Thus, there is a reasonable likelihood that our large sites are population sources, or, as with our small sites, at least very close to the source-sink threshold.
The λ values we estimated (Table 5) are higher than those found in other studies of ovenbirds in more fragmented agricultural landscapes (Donovan et al. 1995), similar to those found in unfragmented forest (Podolsky et al. 2007; λmean ranged from 0.819 to 0.996 using a value of 0.623 for adult survivorship), and lower than those found in edge and interior habitats in a forested landscape (Flaspohler et al. 2001; λ = 1.11 and 1.18, respectively, when using values of 0.60 for adult survivorship and 0.30 for juvenile survivorship). Donovan et al. (1995) found that the forest fragments in their study areas in Missouri and Wisconsin/Minnesota (average forest size > 500 hectares) were well below the source-sink threshold (λ = 0.74 and 0.59, respectively) using a value of 0.623 for adult survivorship. Similarly, Porneluzi and Faaborg (1999) found that forest fragments > 2000 hectares would be population sinks, with female survival at 0.61 and juvenile survival anywhere below 0.56. These comparisons of results from studies of ovenbirds in other landscapes lend further support to the conclusion that the relatively high regional forest cover and irregularly patterned suburban landscape of our study area contribute to greater ovenbird population viability than in forests of similar and even greater size in less forested agricultural landscapes.
The overall nest predation rate of 77% was slightly lower than values found by King et al. (2006) and by Podolsky et al. (2007), 80% and 84%, respectively. Mattsson and Niemi (2006) and Stodola et al. (2010) found higher rates of nest predation (93.3% and 95.7%, respectively) in contiguously forested landscapes, consistent with the suggestion that predation rates may be lower in urbanized areas (Gering and Blair 1999).
Causes of nest failure in our study were similar to those in other studies of ovenbirds (Donovan et al. 1995, Porneluzi and Faaborg 1999). However, brood parasitism rates were much lower in our study sites than in fragmented landscapes in the Midwest. For example, Porneluzi and Faaborg (1999) found that 72% of the nests in a fragmented landscape in Missouri were parasitized, resulting in complete reproductive failure for 31% of the parasitized nests. By contrast, only 29% of the nests in our landscape suffered from cowbird parasitism, and only two (3.2%) of these resulted in complete reproductive failure. Similarly, Porneluzi et al. (1993) found that cowbird parasitism was not a major factor in ovenbird reproductive success in forest fragments in eastern Pennsylvania, and Mattsson and Niemi (2006) found much lower rates of parasitism (6.1%) in forested landscapes in Minnesota. Cowbird densities are much lower in the Northeast than in the Midwest (although their numbers are increasing, Lowther 1993), which is likely one reason that parasitism rates are lower in our study sites than in midwestern sites. In addition, the landscape factors that may make our study sites less permeable to edge predators, namely high forest cover and high connectivity between forests, would also make them less permeable to cowbirds (Hersek et al. 2002).
A suite of factors operating across multiple scales likely influences ovenbird breeding success. Our results are consistent with the suggestion that location within the geographic range (Villard et al. 1993) and regional variation in landscape (Robinson et al. 1995; Bayne and Hobson 2001a; Donnelly and Marzluff 2004) are important considerations, pointing to the need for regional studies to determine minimum area requirements for this species. Forest size is also important: Within the forested suburban landscape we studied, ovenbirds experienced lower reproductive (but not pairing) success in small reserves, and the overall metapopulation was just above or very near the source-sink threshold, with large reserves > 120 hectares being most important and small reserves < 60 hectares hovering at or just below the threshold, although more precise estimates of adult and juvenile survival are still needed (Flaspohler et al. 2001; Podolsky et al. 2007). Our results are consistent with the cutoffs suggested by Donnelly and Marzluff (2004) for conserving native bird communities in urban regions (reserves > 42 hectares in landscapes with > 40% urban land cover), and they support the conservation of small woodlots close to larger forests within landscapes to maintain populations of ovenbirds (Nol et al. 2005).
Within our study site, several factors other than reserve size and regional forest area likely play roles in determining patterns of ovenbird reproductive success. The much higher densities and smaller territory sizes we found in large reserves suggest that large reserves provide superior habitat for ovenbirds, although we found no correlations of density with productivity (Mattsson and Niemi 2008). Ecological traps in lower quality habitats might be swamping density effects (Pérot and Villard 2009). We did perform exploratory analyses of territory vegetation, comparing vegetation in 30 territories in successful (n = 17) and unsuccessful (n = 13) territories, but we found no compelling differences (46 variables measured in ten 0.4-hectare plots within 30 territories), with the possible exception of increased % cover of saplings > 1.0 meter in height in successful territories (unpublished data). Further studies comparing territory vegetation in large vs. small reserves or focusing on microhabitats for fledglings (King et al. 2006; Mattsson and Niemi 2006), nest sites (Reale and Blair 2005), and variations in food abundance (Burke and Nol 1998; Mazerolle and Hobson 2002; Seagle and Sturtevant 2005; Stodola et al. 2010) are needed to determine the effects of habitat quality on breeding success.
Although nest predation was considerable in our study, we found no evidence for differences in nest predation or parasitism rates between large and small forests that might have contributed to differences in reproductive success.
Social factors and social disruption are additional factors that might play roles in influencing the patterns of ovenbird reproductive success in our study and elsewhere (Blumstein and Fernández-Juricic 2004). Bayne and Hobson (2001a) suggest that non-breeding males might have different strategies (floater vs. territorial) in different landscapes, and found that unsuccessful males tend to move longer distances than successful males (2001b). Noise pollution has also been shown to compromise pairing success (Habib et al. 2007) and likely contributed to a lack of pairing success in one site (TP) bordering the Massachusetts Turnpike. Ovenbirds tend to nest in loose colonies (Van Horn 1994), which might allow males to know their neighbors and thus limit their energy expenditures in defending against extra-pair copulation attempts. Fragmentation could affect this system, resulting in greater energy expenditures by males in smaller forests, potentially further strained by limited food availability or suboptimal habitats.
Species exhibit nonlinear thresholds in their responses to forest fragmentation and landscape structure, and thus it is essential that species' responses to landscape change be identified in order to manage for minimum area requirements (Zuckerberg and Porter 2010). Small changes in patch size, forest cover, or connectivity could push species of interest across the thresholds needed to sustain viable populations, as mediated through any number of interacting factors affecting reproductive success, such as changes in predation rates, habitat quality, food availability, or energy expenditure. In suburban landscapes such as the one we studied, maintaining or increasing connectivity among forests fragments and managing the landscape to maximize the amount of canopy cover in residential, private, and conservation areas could maintain populations over the source-sink threshold. We recommend that, ideally, forest cover should be maintained (at least 40%), reserve size maximized (> 120 hectares) and fragmentation minimized in order to support viable ovenbird populations in suburban landscapes in the northeastern U.S.
Urbanization accounts for the majority of developed land in many areas in the Northeast (e.g., Steel 1999). Given this trend, it is of critical importance to recognize the value of the remaining forested habitats in these regions and to manage these landscapes in ways that will maximize their benefits to natural communities and species of interest. We recommend that municipal leaders, land managers, and planners take account of geographic location and regional landscape context when interpreting and then applying results of scientific studies to the management and conservation of viable bird populations in urban regions and elsewhere.
Acknowledgments
We thank J. Moran, R. Harrigan, D. Guillot, J. Lawrence, D. Chow, and numerous undergraduates for assistance in the field, and Y. Federowicz for providing the GIS measurements. We also thank Paul Porneluzi, Frank Thompson III, and Marc-Andre Villard, who read an earlier version of the manuscript. Funding for the research was provided by Regis College, Association of Field Ornithologists, Sigma Xi, and the William Blake Fund of the Nuttall Ornithological Society.